Antiseizure Medication Use and Outcomes After Suspected or Confirmed Acute Symptomatic Seizures
- PMID: 39312247
- PMCID: PMC11420826
- DOI: 10.1001/jamaneurol.2024.3189
Antiseizure Medication Use and Outcomes After Suspected or Confirmed Acute Symptomatic Seizures
Abstract
Importance: Antiseizure medications (ASMs) are frequently prescribed for acute symptomatic seizures and epileptiform abnormalities (EAs; eg, periodic or rhythmic patterns). There are limited data on factors associated with ASM use and their association with outcomes.
Objectives: To determine factors associated with ASM use in patients with confirmed or suspected acute symptomatic seizures undergoing continuous electroencephalography, and to explore the association of ASMs with outcomes.
Design, setting, and participants: This multicenter cohort study was performed between July 1 and September 30, 2021, at 5 US centers of the Post Acute Symptomatic Seizure Investigation and Outcomes Network. After screening 1717 patients, the study included 1172 hospitalized adults without epilepsy who underwent continuous electroencephalography after witnessed or suspected acute symptomatic seizures. Data analysis was performed from November 14, 2023, to February 2, 2024.
Exposure: ASM treatment (inpatient ASM continuation ≥48 hours).
Main outcomes and measures: Factors associated with (1) ASM treatment, (2) discharge ASM prescription, and (3) discharge and 3-month Glasgow Outcome Scale score of 4 or 5 were ascertained.
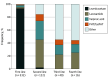
Results: A total of 1172 patients (median [IQR] age, 64 [52-75] years; 528 [45%] female) were included. Among them, 285 (24%) had clinical acute symptomatic seizures, 107 (9%) had electrographic seizures, and 364 (31%) had EAs; 532 (45%) received ASM treatment. Among 922 patients alive at discharge, 288 (31%) were prescribed ASMs. The respective frequencies of inpatient ASM treatment and discharge prescription were 82% (233 of 285) and 69% (169 of 246) for patients with clinical acute symptomatic seizures, 96% (103 of 107) and 95% (61 of 64) for electrographic seizures, and 64% (233 of 364) and 48% (128 of 267) for EAs. On multivariable analysis, acute and progressive brain injuries were independently associated with increased odds of inpatient ASM treatment (odds ratio [OR], 3.86 [95% CI, 2.06-7.32] and 8.37 [95% CI, 3.48-20.80], respectively) and discharge prescription (OR, 2.26 [95% CI, 1.04-4.98] and 10.10 [95% CI, 3.94-27.00], respectively). Admission to the neurology or neurosurgery service (OR, 2.56 [95% CI, 1.08-6.18]) or to the neurological intensive care unit (OR, 7.98 [95% CI, 3.49-19.00]) was associated with increased odds of treatment. Acute symptomatic seizures and EAs were significantly associated with increased odds of ASM treatment (OR, 14.30 [95% CI, 8.52-24.90] and 2.30 [95% CI, 1.47-3.61], respectively) and discharge prescription (OR, 12.60 [95% CI, 7.37-22.00] and 1.72 [95% CI, 1.00-2.97], respectively). ASM treatment was not associated with outcomes at discharge (OR, 0.96 [95% CI, 0.61-1.52]) or at 3 months after initial presentation (OR, 1.26 [95% CI, 0.78-2.04]). Among 623 patients alive and with complete data at 3 months after discharge, 30 (5%) had postdischarge seizures, 187 (30%) were receiving ASMs, and 202 (32%) had all-cause readmissions.
Conclusions and relevance: This study suggests that etiology and electrographic findings are associated with ASM treatment for acute symptomatic seizures and EAs; ASM treatment was not associated with functional outcomes. Comparative effectiveness studies are indicated to identify which patients may benefit from ASMs and to determine the optimal treatment duration.
Conflict of interest statement
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

