Evaluating the potential of non-immunosuppressive cyclosporin analogs for targeting Toxoplasma gondii cyclophilin: Insights from structural studies
- PMID: 39312281
- PMCID: PMC11418636
- DOI: 10.1002/pro.5157
Evaluating the potential of non-immunosuppressive cyclosporin analogs for targeting Toxoplasma gondii cyclophilin: Insights from structural studies
Abstract
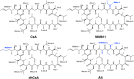
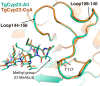
Toxoplasmosis persists as a prevalent disease, facing challenges from parasite resistance and treatment side effects. Consequently, identifying new drugs by exploring novel protein targets is essential for effective intervention. Cyclosporin A (CsA) possesses antiparasitic activity against Toxoplasma gondii, with cyclophilins identified as possible targets. However, CsA immunosuppressive nature hinders its use as an antitoxoplasmosis agent. Here, we evaluate the potential of three CsA derivatives devoid of immunosuppressive activity, namely, NIM811, Alisporivir, and dihydrocyclosporin A to target a previously characterized cyclophilin from Toxoplasma gondii (TgCyp23). We determined the X-ray crystal structures of TgCyp23 in complex with the three analogs and elucidated their binding and inhibitory properties. The high resolution of the structures revealed the precise positioning of ligands within the TgCyp23 binding site and the details of protein-ligand interactions. A comparison with the established ternary structure involving calcineurin indicates that substitutions at position 4 in CsA derivatives prevent calcineurin binding. This finding provides a molecular explanation for why CsA analogs can target Toxoplasma cyclophilins without compromising the human immune response.
Keywords: Toxoplasma gondii; X‐ray crystal structure; cyclophilin inhibitors; cyclophilins; cyclosporin A; peptidyl‐prolyl isomerases.
© 2024 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Structural Basis for Cyclosporin Isoform-Specific Inhibition of Cyclophilins from Toxoplasma gondii.ACS Infect Dis. 2023 Feb 10;9(2):365-377. doi: 10.1021/acsinfecdis.2c00566. Epub 2023 Jan 18. ACS Infect Dis. 2023. PMID: 36653744 Free PMC article.
-
Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology.Antimicrob Agents Chemother. 1997 Sep;41(9):1859-66. doi: 10.1128/AAC.41.9.1859. Antimicrob Agents Chemother. 1997. PMID: 9303374 Free PMC article.
-
Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin.Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13522-6. doi: 10.1073/pnas.212504399. Epub 2002 Sep 30. Proc Natl Acad Sci U S A. 2002. PMID: 12357034 Free PMC article.
-
A Functional Analysis of the Cyclophilin Repertoire in the Protozoan Parasite Trypanosoma Cruzi.Biomolecules. 2018 Oct 31;8(4):132. doi: 10.3390/biom8040132. Biomolecules. 2018. PMID: 30384485 Free PMC article. Review.
-
Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host-parasite interaction and antiparasitic drug action.Int J Parasitol. 2006 Mar;36(3):261-76. doi: 10.1016/j.ijpara.2005.11.003. Epub 2005 Dec 7. Int J Parasitol. 2006. PMID: 16443228 Review.
References
-
- Aebi JD, Deyo DT, Sun CQ, Guillaume D, Dunlap B, Rich DH. Synthesis, conformation, and immunosuppressive activities of three analogues of cyclosporin a modified in the 1‐position. J Med Chem. 1990;33(3):999–1009. - PubMed
-
- Astegno A, Maresi E, Bertoldi M, La Verde V, Paiardini A, Dominici P. Unique substrate specificity of ornithine aminotransferase from toxoplasma gondii. Biochem J. 2017;474(6):939–955. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

