Matrix metalloproteinases mediate influenza A-associated shedding of the alveolar epithelial glycocalyx
- PMID: 39312544
- PMCID: PMC11419339
- DOI: 10.1371/journal.pone.0308648
Matrix metalloproteinases mediate influenza A-associated shedding of the alveolar epithelial glycocalyx
Abstract
Background: The alveolar epithelium is protected by a heparan sulfate-rich, glycosaminoglycan layer called the epithelial glycocalyx. It is cleaved in patients with acute respiratory distress syndrome (ARDS) and in murine models of influenza A (IAV) infection, shedding fragments into the airspace from the cell surface. Glycocalyx shedding results in increased permeability of the alveolar-capillary barrier, amplifying acute lung injury. The mechanisms underlying alveolar epithelial glycocalyx shedding in IAV infection are unknown. We hypothesized that induction of host sheddases such as matrix metalloproteinases (MMPs) during IAV infection results in glycocalyx shedding and increased lung injury.
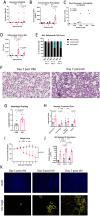
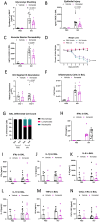
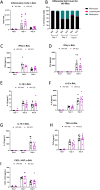
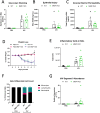
Materials and methods: We measured glycocalyx shedding and lung injury during IAV infection with and without treatment with the pan-MMP inhibitor Ilomastat (ILO) and in an MMP-7 knock out (MMP-7KO) mouse. C57BL/6 or MMP-7KO male and female mice were given IAV A/PR/8/34 (H1N1) at 30,000 PFU/mouse or PBS intratracheally. For some experiments, C56BL/6 mice were infected in the presence of ILO (100mg/kg) or vehicle given daily by IP injection. Bronchoalveolar lavage (BAL) and lung tissue were collected on day 1, 3, and 7 for analysis of glycocalyx shedding (BAL Syndecan-1) and lung injury (histology, BAL protein, BAL cytokines, BAL immune cell infiltrates, BAL RAGE). Expression and localization of the sheddase MMP-7 and its inhibitor TIMP-1 was examined by RNAScope. For in vitro experiments, MLE-12 mouse lung epithelial cells were cultured and treated with active or heat-inactivated heparinase (2.5 U/mL) prior to infection with IAV (MOI 1) and viral load and MMP-7 and TIMP-1 expression analyzed.
Results: IAV infection caused shedding of the epithelial glycocalyx into the BAL. Inhibition of MMPs with ILO reduced glycocalyx shedding by 36% (p = 0.0051) and reduced lung epithelial injury by 40% (p = 0.0404). ILO also reduced viral load by 68% (p = 0.027), despite having no significant effect on lung cytokine production. Both MMP-7 and its inhibitor TIMP-1 were upregulated in IAV infected mice: MMP-7 colocalized with IAV, while TIMP-1 was limited to cells adjacent to infection. However, MMP-7KO mice had similar glycocalyx shedding, epithelial injury, and viral load compared to WT littermates, suggesting redundancy in MMP sheddase function in the lung. In vitro, heparinase treatment before infection led to a 52% increase in viral load (p = 0.0038) without altering MMP-7 or TIMP-1 protein levels.
Conclusions: Glycocalyx shedding and MMPs play key roles in IAV-induced epithelial injury, with significant impact on IAV viral load. Further studies are needed to understand which specific MMPs regulate lung epithelial glycocalyx shedding.
Copyright: © 2024 Schaaf et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Center for Disease Control and Prevention. Past Seasons Estimated Influenza Disease Burden 2024 [https://www.cdc.gov/flu/about/burden/past-seasons.html.
-
- Timm S, Lettau M, Hegermann J, Rocha ML, Weidenfeld S, Fatykhova D, et al. The unremarkable alveolar epithelial glycocalyx: a thorium dioxide-based electron microscopic comparison after heparinase or pneumolysin treatment. Histochem Cell Biol. 2023;160(2):83–96. doi: 10.1007/s00418-023-02211-7 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

