Septo-dentate gyrus cholinergic circuits modulate function and morphogenesis of adult neural stem cells through granule cell intermediaries
- PMID: 39312657
- PMCID: PMC11459179
- DOI: 10.1073/pnas.2405117121
Septo-dentate gyrus cholinergic circuits modulate function and morphogenesis of adult neural stem cells through granule cell intermediaries
Abstract
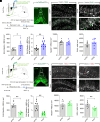
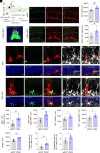
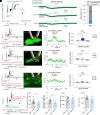
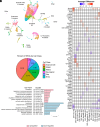
Cholinergic neurons in the basal forebrain play a crucial role in regulating adult hippocampal neurogenesis (AHN). However, the circuit and molecular mechanisms underlying cholinergic modulation of AHN, especially the initial stages of this process related to the generation of newborn progeny from quiescent radial neural stem cells (rNSCs), remain unclear. Here, we report that stimulation of the cholinergic circuits projected from the diagonal band of Broca (DB) to the dentate gyrus (DG) neurogenic niche promotes proliferation and morphological development of rNSCs, resulting in increased neural stem/progenitor pool and rNSCs with longer radial processes and larger busy heads. Interestingly, DG granule cells (GCs) are required for DB-DG cholinergic circuit-dependent modulation of proliferation and morphogenesis of rNSCs. Furthermore, single-nucleus RNA sequencing of DG reveals cell type-specific transcriptional changes in response to cholinergic circuit stimulation, with GCs (among all the DG niche cells) exhibiting the most extensive transcriptional changes. Our findings shed light on how the DB-DG cholinergic circuits orchestrate the key niche components to support neurogenic function and morphogenesis of rNSCs at the circuit and molecular levels.
Keywords: adult neural stem cells; cholinergic circuit; dentate gyrus; diagonal band of Broca; granule cells.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Winkler J., Suhr S. T., Gage F. H., Thal L. J., Fisher L. J., Essential role of neocortical acetylcholine in spatial memory. Nature 375, 484–487 (1995). - PubMed
-
- Green A., et al. , Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol. Biochem. Behav. 81, 575–584 (2005). - PubMed
-
- Buccafusco J. J., Letchworth S. R., Bencherif M., Lippiello P. M., Long-lasting cognitive improvement with nicotinic receptor agonists: Mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol. Sci. 26, 352–360 (2005). - PubMed
MeSH terms
Grants and funding
- R21 NS104530/NS/NINDS NIH HHS/United States
- R01 MH111773/MH/NIMH NIH HHS/United States
- R35 NS097370/NS/NINDS NIH HHS/United States
- F31 AG067718/AG/NIA NIH HHS/United States
- T32 NS007431/NS/NINDS NIH HHS/United States
- R01 MH132222/MH/NIMH NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- RF1 AG079557/AG/NIA NIH HHS/United States
- U54 HD079124/HD/NICHD NIH HHS/United States
- R21 AG058160/AG/NIA NIH HHS/United States
- R01 MH122692/MH/NIMH NIH HHS/United States
- R35 NS116843/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

