A sensitive assay for measuring whole-blood responses to type I IFNs
- PMID: 39312669
- PMCID: PMC11459193
- DOI: 10.1073/pnas.2402983121
A sensitive assay for measuring whole-blood responses to type I IFNs
Abstract
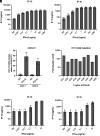
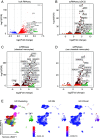
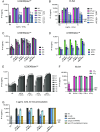
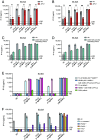
Human inborn errors of the type I IFN response pathway and auto-Abs neutralizing IFN-α, -β, and/or -ω can underlie severe viral illnesses. We report a simple assay for the detection of both types of condition. We stimulate whole blood from healthy individuals and patients with either inborn errors of type I IFN immunity or auto-Abs against type I IFNs with glycosylated human IFN-α2, -β, or -ω. As controls, we add a monoclonal antibody (mAb) blocking the type I IFN receptors and stimulated blood with IFN-γ (type II IFN). Of the molecules we test, IP-10 (encoded by the interferon-stimulated gene (ISG) CXCL10) is the molecule most strongly induced by type I and type II IFNs in the whole blood of healthy donors in an ELISA-like assay. In patients with inherited IFNAR1, IFNAR2, TYK2, or IRF9 deficiency, IP-10 is induced only by IFN-γ, whereas, in those with auto-Abs neutralizing specific type I IFNs, IP-10 is also induced by the type I IFNs not neutralized by the auto-Abs. The measurement of type I and type II IFN-dependent IP-10 induction therefore constitutes a simple procedure for detecting rare inborn errors of the type I IFN response pathway and more common auto-Abs neutralizing type I IFNs.
Keywords: ELISA; IP-10; auto-antibodies; diagnostic test; type I interferons.
Conflict of interest statement
Competing interests statement:J.L.-C. is an inventor on patent application PCT/US2021/042741, filed July 22, 2021, submitted by The Rockefeller University and covering the diagnosis of susceptibility to, and the treatment of, viral disease, and viral vaccines, including COVID-19 and vaccine-associated diseases. Reviewer S.R.O. is a co-author of two recent papers with J.L.C. Reviewer P.H. has a collaboration with the Casanova group in the exchange of reagents, but it does not cover the topic of this collaboration.
Figures
References
-
- Casanova J.-L., Ion gresser. J. Interferon Cytokine Res. 39, 317–320 (2019), 10.1089/jir.2018.29015.mem. - DOI
MeSH terms
Substances
Grants and funding
- ANR-22-CE92-0004/Agence Nationale de la Recherche (ANR)
- N/A/JPB Foundation (JPBF)
- N/A/Fondation Bettencourt Schueller (Bettencourt Schueller Foundation)
- N/A/Meyer Foundation
- N/A/King Abdullah University of Science and Technology (KAUST)
- N/A/Fondation du Souffle (FdS)
- N/A/General Atlantic Foundation
- N/A/Battersea & Bowery Advisory Group
- ANR-10-LABX-62-IBEID/Agence Nationale de la Recherche (ANR)
- R01 AI127564/AI/NIAID NIH HHS/United States
- ANR-21-RHUS-08/Agence Nationale de la Recherche (ANR)
- MESRI-COVID-19/French Ministry of Higher Education, Research, and Innovation
- ANR-21-LIBA-0002/Agence Nationale de la Recherche (ANR)
- N/A/Grandir - Fonds de solidarité pour l'enfance
- N/A/Fisher Center for Alzheimer's Research Foundation (Fisher Center)
- N/A/Stavros Niarchos Foundation (SNF)
- R01AI163029/HHS | National Institutes of Health (NIH)
- R01 AI095983/AI/NIAID NIH HHS/United States
- UL1TR001866/HHS | NIH | National Center for Advancing Translational Sciences (NCATS)
- N/A/King Abdulaziz City for Science and Technology (KACST)
- EA20 170638020/Fondation pour la Recherche Médicale (FRM)
- ANR-22-CE15-0046/Agence Nationale de la Recherche (ANR)
- 01057100 (UNDINE)/EC | HORIZON EUROPE Framework Programme (Horizon Europe)
- UL1 TR001866/TR/NCATS NIH HHS/United States
- EA20170638020/Fondation pour la Recherche Médicale (FRM)
- ANR-20-CE93-003/Agence Nationale de la Recherche (ANR)
- ANR-10-IAHU-01/Agence Nationale de la Recherche (ANR)
- N/A/Assistance Publique Hopitaux de Paris
- R01AI127564/HHS | National Institutes of Health (NIH)
- ANR - 10 - LABX - 62 - 01/Agence Nationale de la Recherche (ANR)
- R01 AI163029/AI/NIAID NIH HHS/United States
- N/A/Square Foundation
- N/A/SCOR Corporate Foundation for Science (SCOR Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

