Glutathione peroxidase 3 is essential for countering senescence in adipose remodelling by maintaining mitochondrial homeostasis
- PMID: 39312866
- PMCID: PMC11447410
- DOI: 10.1016/j.redox.2024.103365
Glutathione peroxidase 3 is essential for countering senescence in adipose remodelling by maintaining mitochondrial homeostasis
Abstract
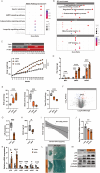
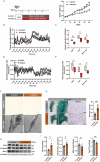
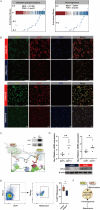
Adipose tissue senescence is a precursor to organismal aging and understanding adipose remodelling contributes to discovering novel anti-aging targets. Glutathione peroxidase 3 (GPx3), a critical endogenous antioxidant enzyme, is diminished in the subcutaneous adipose tissue (sWAT) with white adipose expansion. Based on the active role of the antioxidant system in counteracting aging, we investigated the involvement of GPx3 in adipose senescence. We determined that knockdown of GPx3 in adipose tissue by adeno-associated viruses impaired mitochondrial function in mice, increased susceptibility to obesity, and exacerbated adipose tissue senescence. Impairment of GPx3 may cause mitochondrial dysfunction through inner mitochondrial membrane disruption. Adipose reshaping management (cold stimulation and intermittent diet) counteracted the aging of tissues, with an increase in GPx3 expression. Overall metabolic improvement induced by cold stimulation was partially attenuated when GPx3 was depleted. GPx3 may be involved in adipose browning by interacting with UCP1, and GPx3 may be a limiting factor for intracellular reactive oxygen species (ROS) accumulation during stem cell browning. Collectively, these findings emphasise the importance of restoring the imbalanced redox state in adipose tissue to counteract aging and that GPx3 may be a potential target for maintaining mitochondrial homeostasis and longevity.
Keywords: Adipose tissue; Anti-Senescence; Glutathione peroxidase 3; Mitochondrial.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None. The authors declare that there are no known financial interests or personal relationships that might interfere with work reported in this paper.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

