Aging disrupts locus coeruleus-driven norepinephrine transmission in the prefrontal cortex: Implications for cognitive and motor decline
- PMID: 39312903
- PMCID: PMC11709105
- DOI: 10.1111/acel.14342
Aging disrupts locus coeruleus-driven norepinephrine transmission in the prefrontal cortex: Implications for cognitive and motor decline
Abstract
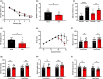
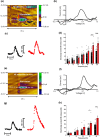
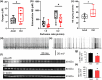
The locus coeruleus (LC)-prefrontal cortex (PFC) circuitry is crucial for cognition, planning, posture and mobility. This study examines the role of norepinephrine (NE) in elucidating the neurobiological basis of age-related cognitive and motor declines. Aged mice exhibited reduced spatial learning, impaired memory, decreased physical endurance, and notable changes in locomotor behavior. The neurochemical foundations of these deficits were investigated through fast-scan cyclic voltammetry to measure NE release in the PFC and LC, both in vivo and in brain slices. Additionally, oxygen levels were monitored as a proxy for PFC neuronal function, and NE levels were analyzed in the extracellular space via microdialysis and total content in the PFC. Aged mice exhibited a frequency-dependent increase in NE release in the PFC upon LC stimulation, suggesting alterations in neural responsiveness due to aging. We also recorded slower NE reuptake rates and increased NE content and neuronal activity, indicated by higher oxygen levels and facilitated neuron activation due to membrane depolarization recorded via whole-cell patch-clamp. To understand the basis for LC-driven NE surges in the PFC with aging, we examined the expression levels of two proteins critical for presynaptic NE release and NE reuptake: the α2a-adrenergic receptor and the NE transporter. Both showed a significant decrease in the PFC with aging. These findings support the concept that aging significantly alters the structural and functional dynamics within the LC-PFC neural circuit, impacting NE modulation and neuronal activity, which may underlie the observed declines in cognitive and motor functions in aging populations.
Keywords: Gait; aging; locus coeruleus; memory; mobility; noradrenergic neurons; prefrontal cortex.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bruinstroop, E. , Cano, G. , Vanderhorst, V. G. J. M. , Cavalcante, J. C. , Wirth, J. , Sena‐Esteves, M. , & Saper, C. B. (2012). Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. The Journal of Comparative Neurology, 520(9), 1985–2001. 10.1002/cne.23024 - DOI - PMC - PubMed
-
- Buchman, A. S. , Boyle, P. A. , Leurgans, S. E. , Barnes, L. L. , & Bennett, D. A. (2011). Cognitive function is associated with the development of mobility impairments in community‐dwelling elders. The American Journal of Geriatric Psychiatry, 19(6), 571–580. 10.1097/JGP.0b013e3181ef7a2e - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

