Value for money of medicine sampling and quality testing: evidence from Indonesia
- PMID: 39313254
- PMCID: PMC11429347
- DOI: 10.1136/bmjgh-2024-015402
Value for money of medicine sampling and quality testing: evidence from Indonesia
Abstract
Background: Substandard and falsified medicines (SFMs) are a public health concern of global importance. Postmarket surveillance in the form of medicine sampling and quality testing can prevent and detect SFM, however, there is remarkably scarce evidence about the cost and value for money of these activities: how much do they cost and how effective are they in detecting SFM?
Methods: Between February and October 2022, Systematic Tracking of At Risk Medicines (STARmeds) collected and analysed for quality 1274 samples of 5 medicines from physical and online retail outlets in 7 Indonesian districts. We collated data on the resources consumed by STARmeds, related to all stages of medicines sampling and quality testing including design, fieldwork and laboratory analysis. We used activity-based costing principles to calculate the financial and economic cost of medicine quality surveillance from the perspective of a hypothetical medicines' regulator. We calculated the cost per day and per week of fieldwork, per sample collected and per substandard sample. We used bootstrapping to capture uncertainty in the number of samples collected, by seller location type (urban, rural and online).
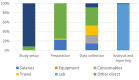
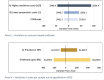
Results: The total cost of sampling and testing medicines from the market was US$712 964 (current 2022 values). Laboratory costs represented the largest share (70%), followed by other direct costs (12%) and indirect costs (7%). On average, it costs STARmeds US$479 (95% CI US$462 to US$516) to collect one medicine sample and US$5990 (95% CI US$5601 to US$6258) to identify one substandard sample.
Conclusion: Our findings bring urgently needed and novel information on the cost and value for money of medicine quality surveillance. These may support planning and budgeting of the Indonesian pharmaceutical regulator, but also of regulators and researchers elsewhere, particularly in low-income and middle-income settings, as well as international organisations with health regulation and quality of care remits.
Keywords: Cross-sectional survey; Global Health; Pharmacology; Public Health.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Irawati R. Pharmacovigilance System and Its Implementation in Indonesia. Japan - Indonesia: 1st Joint Symposium; 2013. https://www.pmda.go.jp/files/000216985.pdf Available.
-
- USAID . USAID; 2018. Strengthening indonesia’s pharmaceutical post-marketing surveillance capacity.
-
- World Health Organization . Geneva:World Health Organization; 2017. WHO global surveillance and monitoring system for substandard and falsified medical products.https://apps.who.int/iris/handle/10665/326708 Available.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
