Cellular pattern and orbital fat involvement are possible risk factors for the failure of corticosteroids in patients with pure idiopathic orbital inflammation syndrome: lessons from the French prospective SIOI cohort study (part II)
- PMID: 39313294
- PMCID: PMC11418487
- DOI: 10.1136/bmjophth-2024-001663
Cellular pattern and orbital fat involvement are possible risk factors for the failure of corticosteroids in patients with pure idiopathic orbital inflammation syndrome: lessons from the French prospective SIOI cohort study (part II)
Abstract
Purpose: To better characterise the effects of corticosteroids on the course of pure idiopathic orbital inflammation syndrome (pIOIS).
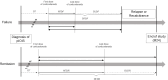
Methods: This was a national, multicentre, prospective, non-interventional cohort study (SIOI). Among the 35 patients with histologically proven orbital inflammation who had previously been studied for their IgG4 immunostaining status, we selected those with a negative IgG4 status (ie, pIOIS) who received corticosteroids as single first-line treatment. Clinical, morphological and pathological findings at diagnosis and during follow-up from treatment initiation to study completion were analysed. Patients were assessed for their response to prednisone after the 24-month prospective phase in terms of remission (≤10 mg/d) or failure (>10 mg/d). Daily standard doses of prednisone (DSDP) were calculated at different time-points and compared between response groups.
Results: Of the 17 patients with pIOIS included in the final analysis, two-thirds received corticosteroids only. DSDP (mg/kg-day) were significantly higher at the time of failure in eight patients (47%) than in nine (53%) remitting at M24 (0.16 vs 0.045; p: 0.03). Notably, patients with pIOIS with a cellular pattern or orbital fat involvement tended to receive higher daily corticosteroid doses in the event of failure than remission (0.16 vs 0.045 and 0.12 vs 0.042, respectively). During treatment, maximal DSDP was 0.52 in failed patients.
Conclusion: The highest corticosteroid doses were insufficient to prevent failure in patients with pIOIS, particularly in those with a cellular pattern or orbital fat involvement. Large-scale interventional studies are now necessary to clarify prognostic factors and optimise corticosteroid management in patients with pIOIS.
Keywords: Immunology; Inflammation; Lacrimal gland; Orbit; Treatment Medical.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
