BCMA-BBZ-OX40 CAR-T Therapy Using an Instant Manufacturing Platform in Multiple Myeloma
- PMID: 39313307
- PMCID: PMC11418555
- DOI: 10.1136/jitc-2024-009476
BCMA-BBZ-OX40 CAR-T Therapy Using an Instant Manufacturing Platform in Multiple Myeloma
Abstract
Background: Chimeric antigen receptor (CAR)-T cell has revolutionary efficacy against relapsed/refractory multiple myeloma (R/R MM). However, current CAR-T cell therapy has several limitations including long vein-to-vein time and limited viability.
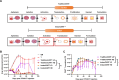
Methods: A 4-1BB-costimulated B-cell maturation antigen (BCMA) CAR-T integrating an independently-expressed OX40 (BCMA-BBZ-OX40) was designed and generated by a traditional manufacturing process (TraditionCART) or instant manufacturing platform (named InstanCART). The tumor-killing efficiency, differentiation, exhaustion, and expansion level were investigated in vitro and in tumor-bearing mice. An investigator-initiated clinical trial was performed in patients with R/R MM to evaluate the outcomes of both TraditionCART and InstanCART. The primary objective was safety within 1 month after CAR-T cell infusion. The secondary objective was the best overall response rate.
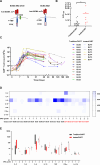
Results: Preclinical studies revealed that integrated OX40 conferred BCMA CAR-T cells with superior cytotoxicity and reduced exhaustion levels. InstanCART process further enhanced the proliferation and T-cell stemness of BCMA-BBZ-OX40 CAR-T cells. BCMA-BBZ-OX40 CAR-T cells were successfully administered in 22 patients with R/R MM, including 15 patients with TraditionCART and 7 patients with InstanCART. Up to 50% (11/22) patients had a high-risk cytogenetic profile and 36% (8/22) had extramedullary disease. CAR-T therapy caused grade 1-2 cytokine release syndrome in 19/22 (80%) patients, grade 1 neurotoxicity in 2/22 (9%) patients and led to ≥grade 3 adverse events including neutropenia (20/22, 91%), thrombocytopenia (15/22, 68%), anemia (12/22, 55%), creatinine increased (1/22, 5%), hepatic enzymes increased (5/22, 23%), and sepsis (1/22, 5%). The best overall response rate was 100%, and 64% (14/22) of the patients had a complete response or better. The median manufacturing time was shorter for InstanCART therapy (3 days) than for TraditionCART therapy (10 days). Expansion and duration were dramatically higher for InstanCART cells than for TraditionCART cells.
Conclusions: BCMA-BBZ-OX40 CAR-T cells were well tolerated and exhibited potent responses in patients with R/R MM. InstanCART shortened the manufacturing period compared to TraditionCART, and improved the cellular kinetics. Our results demonstrated the potency and feasibility of OX40-modified BCMA CAR-T cells using InstanCART technology for R/R MM therapy.
Trial registration number: This trial was registered at www.
Clinicaltrials: gov as #NCT04537442.
Keywords: Chimeric Antigen Receptor - CAR; Hematologic Malignancies; Immunotherapy; Multiple Myeloma; T cell.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
