A Prefrontal→Periaqueductal Gray Pathway Differentially Engages Autonomic, Hormonal, and Behavioral Features of the Stress-Coping Response
- PMID: 39313320
- PMCID: PMC11561873
- DOI: 10.1523/JNEUROSCI.0844-24.2024
A Prefrontal→Periaqueductal Gray Pathway Differentially Engages Autonomic, Hormonal, and Behavioral Features of the Stress-Coping Response
Abstract
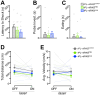
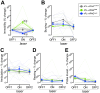
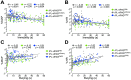
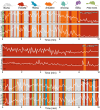
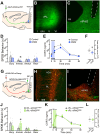
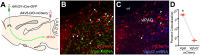
The activation of autonomic and hypothalamo-pituitary-adrenal (HPA) systems occurs interdependently with behavioral adjustments under varying environmental demands. Nevertheless, laboratory rodent studies examining the neural bases of stress responses have generally attributed increments in these systems to be monolithic, regardless of whether an active or passive coping strategy is employed. Using the shock probe defensive burying test (SPDB) to measure stress-coping features naturalistically in male and female rats, we identify a neural pathway whereby activity changes may promote distinctive response patterns of hemodynamic and HPA indices typifying active and passive coping phenotypes. Optogenetic excitation of the rostral medial prefrontal cortex (mPFC) input to the ventrolateral periaqueductal gray (vlPAG) decreased passive behavior (immobility), attenuated the glucocorticoid hormone response, but did not prevent arterial pressure and heart rate increases associated with rats' active behavioral (defensive burying) engagement during the SPDB. In contrast, inhibition of the same pathway increased behavioral immobility and attenuated hemodynamic output but did not affect glucocorticoid increases. Further analyses confirmed that hemodynamic increments occurred preferentially during active behaviors and decrements during immobility epochs, whereas pathway manipulations, regardless of the directionality of effect, weakened these correlational relationships. Finally, neuroanatomical evidence indicated that the influence of the rostral mPFC→vlPAG pathway on coping response patterns is mediated predominantly through GABAergic neurons within vlPAG. These data highlight the importance of this prefrontal→midbrain connection in organizing stress-coping responses and in coordinating bodily systems with behavioral output for adaptation to aversive experiences.
Keywords: HPA axis; cardiovascular; neural circuit; periaqueductal gray; prelimbic cortex; shock probe defensive burying test.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
