Selective Vulnerability of GABAergic Inhibitory Interneurons to Bilirubin Neurotoxicity in the Neonatal Brain
- PMID: 39313321
- PMCID: PMC11551895
- DOI: 10.1523/JNEUROSCI.0442-24.2024
Selective Vulnerability of GABAergic Inhibitory Interneurons to Bilirubin Neurotoxicity in the Neonatal Brain
Abstract
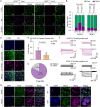
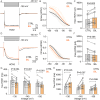
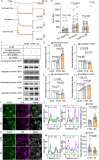
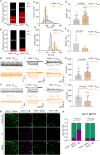
Hyperbilirubinemia (HB) is a key risk factor for hearing loss in neonates, particularly premature infants. Here, we report that bilirubin (BIL)-dependent cell death in the auditory brainstem of neonatal mice of both sexes is significantly attenuated by ZD7288, a blocker for hyperpolarization-activated cyclic nucleotide-gated (HCN) channel-mediated current (I h), or by genetic deletion of HCN1. GABAergic inhibitory interneurons predominantly express HCN1, on which BIL selectively acts to increase their intrinsic excitability and mortality by enhancing HCN1 activity and Ca2+-dependent membrane targeting. Chronic BIL elevation in neonatal mice in vivo increases the fraction of spontaneously active interneurons and their firing frequency, I h, and death, compromising audition at the young adult stage in HCN1+/+, but not in HCN1-/- genotype. We conclude that HB preferentially targets HCN1 to injure inhibitory interneurons, fueling a feedforward loop in which lessening inhibition cascades hyperexcitability, Ca2+ overload, neuronal death, and auditory impairments. These findings rationalize HCN1 as a potential target for managing HB encephalopathy.
Keywords: GABAergic interneurons; auditory impairment; bilirubin; cochlear nucleus neuron; hyperpolarization-activated cyclic nucleotide-gated channels; neurotoxicity.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
