Concurrent validity, cut-offs and ability to change of patient-reported outcome measures for rhinitis and asthma in MASK-air®
- PMID: 39313483
- PMCID: PMC11419846
- DOI: 10.1002/clt2.12390
Concurrent validity, cut-offs and ability to change of patient-reported outcome measures for rhinitis and asthma in MASK-air®
Abstract
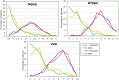
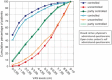
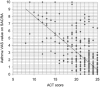
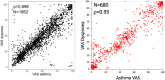
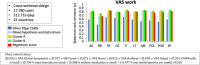
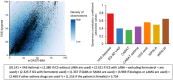
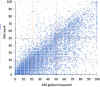
Patient-reported outcome measures (PROMs) are used to assess a patient's health status at a particular point in time. They are essential in the development of person-centred care. This paper reviews studies performed on PROMs for assessing AR and asthma control, in particular VAS scales that are included in the app MASK-air® (Mobile Airways Sentinel networK) for asthma and rhinitis. VASs were initially developed on paper and pencil and tested for their criterion validity, cut-offs and responsiveness. Then, a multicentric, multinational, double-blind, placebo-controlled, randomised control trial (DB-PC-RCT) using an electronic VAS form was carried out. Finally, with the development of MASK-air® in 2015, previously validated VAS questions were adapted to the digital format and further methodologic evaluations were performed. VAS for asthma, rhinitis, conjunctivitis, work and EQ-5D are included in the app. Additionally, two control-medication scores for allergic symptoms of asthma (e-DASTHMA) were validated for their criterion validity, cut-offs and responsiveness.
Keywords: EQ‐5D; asthma; digital health; rhinitis; visual analogue scale.
© 2024 The Author(s). Clinical and Translational Allergy published by John Wiley & Sons Ltd on behalf of European Academy of Allergy and Clinical Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Klimek L, Bergmann KC, Biedermann T, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: position paper of the German society of allergology (AeDA) and the German society of allergy and clinical immunology (DGAKI), ENT section, in collaboration with the working group on clinical immunology, allergology and environmental medicine of the German society of otorhinolaryngology, head and neck surgery (DGHNOKHC). Allergo J Int. 2017;26(1):16‐24. 10.1007/s40629-016-0006-7 - DOI - PMC - PubMed
-
- Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022‐1024. - PubMed
-
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45‐56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

