In vitro comparison of human and murine trabecular meshwork cells: implications for glaucoma research
- PMID: 39313534
- PMCID: PMC11420201
- DOI: 10.1038/s41598-024-73057-9
In vitro comparison of human and murine trabecular meshwork cells: implications for glaucoma research
Abstract
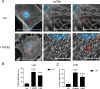
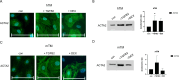
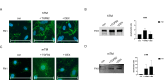
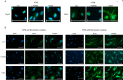
The trabecular meshwork (TM) is crucial for regulating intraocular pressure (IOP), and its dysfunction significantly contributes to glaucoma, a leading cause of vision loss and blindness worldwide. Although rodents are commonly used as animal models in glaucoma research, the applicability of these findings to humans is limited due to the insufficient understanding of murine TM. This study aimed to compare primary human TM (hTM) and murine TM (mTM) cells in vitro to enhance the robustness and translatability of murine glaucoma models. In this in vitro study, we compared primary hTM and mTM cells under simulated physiological and pathological conditions by exposing both cell types to the glucocorticoid dexamethasone (DEX) and Transforming Growth Factor β (TGFB2), both of which are critical in the pathogenesis of several ophthalmological diseases, including glaucoma. Phagocytic properties were assessed using microbeads. Cells were analyzed through immunocytochemistry (ICC) and Western blot (WB) to evaluate the expression of extracellular matrix (ECM) components, such as Fibronectin 1 (FN1) and Collagen IV (COL IV). Filamentous-Actin (F-Act) staining was used to analyze cross-linked actin network (CLAN) formation. Additionally, we evaluated cytoskeletal components, including Vimentin (VIM), Myocilin (MYOC), and Actin-alpha-2 (ACTA2). Our results demonstrated significant similarities between human and murine TM cells in basic morphology, phagocytic properties, and ECM and cytoskeletal component expression under both homeostatic and pathological conditions in vitro. Both human and murine TM cells exhibited epithelial-to-mesenchymal transition (EMT) after exposure to DEX or TGFB2, with comparable CLAN formation observed in both species. However, there were significant differences in FN1 and MYOC induction between human and murine TM cells. Additionally, MYOC expression in hTM cells depended on fibronectin coating. Our study suggests that murine glaucoma models are potentially translatable to human TM. The observed similarities in ECM and cytoskeletal component expression and the comparable EMT response and CLAN formation support the utility of murine models in glaucoma research. The differences in FN1 and MYOC expression between hTM and mTM warrant further investigation due to their potential impact on TM properties. Overall, this study provides valuable insights into the species-specific characteristics of TM and highlights opportunities to refine murine models for better relevance to human glaucoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Johnson, M. & Erickson, K. Mechanisms and routes of aqueous humor drainage. In Principles and Practice of Ophthalmology 2577–2595WB (Saunders Co., 2000).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

