Loss of electrical β-cell to δ-cell coupling underlies impaired hypoglycaemia-induced glucagon secretion in type-1 diabetes
- PMID: 39313541
- PMCID: PMC11599053
- DOI: 10.1038/s42255-024-01139-z
Loss of electrical β-cell to δ-cell coupling underlies impaired hypoglycaemia-induced glucagon secretion in type-1 diabetes
Abstract
Diabetes mellitus involves both insufficient insulin secretion and dysregulation of glucagon secretion1. In healthy people, a fall in plasma glucose stimulates glucagon release and thereby increases counter-regulatory hepatic glucose production. This response is absent in many patients with type-1 diabetes (T1D)2, which predisposes to severe hypoglycaemia that may be fatal and accounts for up to 10% of the mortality in patients with T1D3. In rats with chemically induced or autoimmune diabetes, counter-regulatory glucagon secretion can be restored by SSTR antagonists4-7 but both the underlying cellular mechanism and whether it can be extended to humans remain unestablished. Here, we show that glucagon secretion is not stimulated by low glucose in isolated human islets from donors with T1D, a defect recapitulated in non-obese diabetic mice with T1D. This occurs because of hypersecretion of somatostatin, leading to aberrant paracrine inhibition of glucagon secretion. Normally, KATP channel-dependent hyperpolarization of β-cells at low glucose extends into the δ-cells through gap junctions, culminating in suppression of action potential firing and inhibition of somatostatin secretion. This 'electric brake' is lost following autoimmune destruction of the β-cells, resulting in impaired counter-regulation. This scenario accounts for the clinical observation that residual β-cell function correlates with reduced hypoglycaemia risk8.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
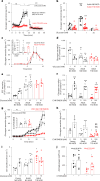



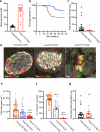



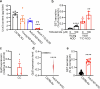

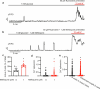



References
-
- Unger, R. H. Glucagon and insulin: a bihormonal system. Compr. Ther.2, 20–26 (1976). - PubMed
-
- Gerich, J. E., Langlois, M., Noacco, C., Karam, J. H. & Forsham, P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science182, 171–173 (1973). - PubMed
-
- Cryer, P. E. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes63, 2188–2195 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- G-1912-03553/Leona M. and Harry B. Helmsley Charitable Trust (Helmsley Charitable Trust)
- 2013-7107/Vetenskapsrådet (Swedish Research Council)
- 14/0005128/DUK_/Diabetes UK/United Kingdom
- G-2305-06047/Leona M. and Harry B. Helmsley Charitable Trust (Helmsley Charitable Trust)
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

