The French registry of sudden unexpected death in infancy (SUDI): a 7-year review of available data
- PMID: 39313586
- PMCID: PMC11473449
- DOI: 10.1007/s00431-024-05727-9
The French registry of sudden unexpected death in infancy (SUDI): a 7-year review of available data
Abstract
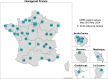
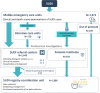
The French "OMIN registry" was established in 2015 to collect nationwide standardised data concerning biological, clinical, environmental and social characteristics of sudden unexpected death in infancy (SUDI) and unexpected death in children aged 1-2 years. A biobank has existed since July 2020 to store biological samples for each case. This article aimed to detail (1) a brief history and the objectives of the registry; (2) a description of the methodology used; (3) the first results of the registry, i.e. the main characteristics of the cases included so far; (4) the process for accessing the data for research projects; and (5) issues regarding weakness and improvement and perspectives offered by the registry. On 31 May 2024, 1975 cases were included in the OMIN registry; on 31 December 2022, 4606 biological samples from 176 cases were collected. For each deceased child, different types of data are registered on an electronic case report form: socio-demographic data, personal and familial medical background, environment and feeding data, clinical data, and biological and imaging results. A strict and continuous quality control process is used to ensure the reliability of the data, in parallel with specific actions to improve the exhaustiveness of the registry. The OMIN registry database is one of the largest and the most complete databases on SUDI, especially in Europe, and the first in the world to associate a standardised biological sample collection with it. Perspectives of research provided by our registry are numerous and could be supported by national and international scientific collaborations.
Conclusion: This article details the objectives and methods of the French registry of SUDI. It provides initial results relating to the population included in the register and the procedure for accessing the data.
What is known: • In Western Europe, France is one of the countries with the highest SUDI rate, making it the first cause of death of infants between 28 and 364 days. • The development of epidemiological tools on a national and international scale is essential to advance research into the determinants and risk factors of unexpected death in children under 2 years of age.
What is new: • The OMIN registry was created in France in 2015 to collect nationwide standardised social, environmental, clinical, and paraclinical data for cases of unexpected death in children aged 0 to 2 years. • To date, the OMIN registry has included 680 data from almost 2000 children unexpectedly deceased, completed by a biocollection since 2020. • Data from the OMIN registry, unique in its field, are freely available for scientific research teams, after acceptation by the scientific committee of the registry.
Keywords: Epidemiological surveillance; Population registry; Public health; SIDS; SUDI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Blair PS, Byard RW, Fleming PJ (2012) Sudden unexpected death in infancy (SUDI): suggested classification and applications to facilitate research activity. Forensic Sci Med Pathol 8:312–315. 10.1007/s12024-011-9294-x - PubMed
-
- Krous HF, Chadwick AE, Crandall L, Nadeau-Manning JM (2005) Sudden unexpected death in childhood: a report of 50 cases. Pediatr Dev Pathol 8:307–319. 10.1007/s10024-005-1155-8 - PubMed
-
- The Royal college of pathologists, The royal college of paediatrics and child health (2016) Sudden unexpected death in infancy and childhood, multi-agency guidelines for care and investigation. second, ed. London, 2016.
-
- Krous HF (2004) Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 114:234–238. 10.1542/peds.114.1.234 - PubMed
-
- de Visme S, Chalumeau M, Levieux K et al (2020) National variations in recent trends of sudden unexpected infant death rate in Western Europe. J Pediatr 226:179-185.e4. 10.1016/j.jpeds.2020.06.052 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

