Innovative biomarkers TCN2 and LY6E can significantly inhibit respiratory syncytial virus infection
- PMID: 39313785
- PMCID: PMC11421179
- DOI: 10.1186/s12967-024-05677-8
Innovative biomarkers TCN2 and LY6E can significantly inhibit respiratory syncytial virus infection
Abstract
Background: Respiratory syncytial virus (RSV) is a prominent etiological agent of lower respiratory tract infections in children, responsible for approximately 80% of cases of pediatric bronchiolitis and 50% of cases of infant pneumonia. Despite notable progress in the diagnosis and management of pediatric RSV infection, the current biomarkers for early-stage detection remain insufficient to meet clinical needs. Therefore, the development of more effective biomarkers for early-stage pediatric respiratory syncytial virus infection (EPR) is imperative.
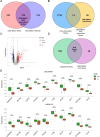
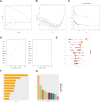
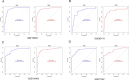
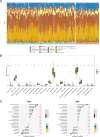
Methods: The datasets used in this study were derived from the Gene Expression Omnibus (GEO) database. We used GSE188427 dataset as the training set to screen for biomarkers. Biomarkers of EPR were screened by Weighted Gene Co-expression Network Analysis (WGCNA), three machine-learning algorithms (LASSO regression, Random Forest, XGBoost), and other comprehensive bioinformatics analysis techniques. To evaluate the diagnostic value of these biomarkers, multiple external and internal datasets were employed as validation sets. Next, an examination was performed to investigate the relationship between the screened biomarkers and the infiltration of immune cells. Furthermore, an investigation was carried out to identify potential small molecule compounds that interact with selected diagnostic markers. Finally, we confirmed that the expression levels of the selected biomarkers exhibited a significant increase following RSV infection, and they were further identified as having antiviral properties.
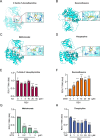
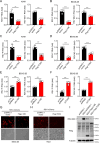
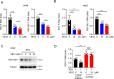
Results: The study found that lymphocyte antigen 6E (LY6E) and Transcobalamin-2 (TCN2) are two biomarkers with diagnostic significance in EPR. Analysis of immune cell infiltration showed that they were associated with activation of multiple immune cells. Furthermore, our analysis demonstrated that small molecules, 3'-azido-3'-deoxythymine, methotrexate, and theophylline, have the potential to bind to TCN2 and exhibit antiviral properties. These compounds may serve as promising therapeutic agents for the management of pediatric RSV infections. Additionally, our data revealed an upregulation of LY6E and TCN2 expression in PBMCs from patients with RSV infection. ROC analysis indicated that LY6E and TCN2 possessed diagnostic value for RSV infection. Finally, we confirmed that LY6E and TCN2 expression increased after RSV infection and further inhibited RSV infection in A549 and BEAS-2B cell lines. Importantly, based on TCN2, our findings revealed the antiviral properties of a potentially efficacious compound, vitamin B12.
Conclusion: LY6E and TCN2 are potential peripheral blood diagnostic biomarkers for pediatric RSV infection. LY6E and TCN2 inhibit RSV infection, indicating that LY6E and TCN2 are potential therapeutic target for RSV infection.
Keywords: Biomarker; LY6E; Pediatric RSV infection; Respiratory syncytial virus (RSV); TCN2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Identification of potential immune/diagnosis related gene-immunocyte subtype networks in extracellular immune response to respiratory syncytial virus infection.Virus Res. 2022 Nov;321:198906. doi: 10.1016/j.virusres.2022.198906. Epub 2022 Aug 28. Virus Res. 2022. PMID: 36044931
-
CONSTRUCTING A DIAGNOSTIC PREDICTION MODEL TO ESTIMATE THE SEVERE RESPIRATORY SYNCYTIAL VIRUS PNEUMONIA IN CHILDREN BASED ON MACHINE LEARNING.Shock. 2025 Apr 1;63(4):533-540. doi: 10.1097/SHK.0000000000002472. Epub 2024 Sep 11. Shock. 2025. PMID: 39283056
-
A novel combined nomogram for predicting severe acute lower respiratory tract infection in children hospitalized for RSV infection during the post-COVID-19 period.Front Immunol. 2024 Jul 24;15:1437834. doi: 10.3389/fimmu.2024.1437834. eCollection 2024. Front Immunol. 2024. PMID: 39114651 Free PMC article.
-
Biomarkers for Disease Severity in Children Infected With Respiratory Syncytial Virus: A Systematic Literature Review.J Infect Dis. 2020 Oct 7;222(Suppl 7):S648-S657. doi: 10.1093/infdis/jiaa208. J Infect Dis. 2020. PMID: 32794555
-
Respiratory syncytial virus: how, why and what to do.J Infect. 2014 Jan;68 Suppl 1:S115-8. doi: 10.1016/j.jinf.2013.09.021. Epub 2013 Oct 27. J Infect. 2014. PMID: 24171820 Review.
References
-
- Guzmán Molina C, Rodríguez-Belvís MV, Coroleu Bonet A, Vall Combelles O, García-Algar O. Antibiotics in respiratory tract infections in hospital pediatric emergency departments. Arch Bronconeumol. 2014;50(9):375–8. - PubMed
-
- Qiu X, Xu S, Lu Y, Luo Z, Yan Y, Wang C, Ji J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 2022;68:37–53. - PubMed
MeSH terms
Substances
Grants and funding
- 82270034/Natural Science Foundation of China
- 82302654/Natural Science Foundation of China
- SBK2023044153/Natural Science Foundation of Jiangsu Province
- BJ2023028/Youth and Middle-aged Scientific and Technological Innovation Leading Talents Program of the Corps
- Q202228/Young Scholars of Wuxi Municipal Health Commission
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

