Development of a Simvastatin-Loaded Copolymer Acid-Sensitive Nanocarrier and Its Experimental Use in the Treatment of Keloids
- PMID: 39313951
- PMCID: PMC11743034
- DOI: 10.1111/jocd.16573
Development of a Simvastatin-Loaded Copolymer Acid-Sensitive Nanocarrier and Its Experimental Use in the Treatment of Keloids
Abstract
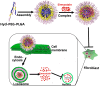
Objective: The lipid-lowering simvastatin (SIM) has been shown to be an effective inhibitor of keloid proliferation. However, due to its low water solubility and short half-life, simvastatin aggregates to the liver and does not reach the skin lesions after oral administration, which restricts its widespread clinical use. The development of nanomedicine provides the possibility for us to break through this bottleneck problem clinically. The objective of this study was to investigate the feasibility of using complex nanocontrolled delivery system (CNDS), simvastatin-loaded polyethylene glycol-poly lactic-co-glycolic acid (PEG-PLGA), in the treatment of keloids.
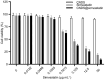
Methods: In the in vitro study, the release of simvastatin in fibroblasts by CNDS@Simvastatin and its effect on inhibition of the proliferation of fibroblasts, Col Ι, and CTGF by the slow release of simvastatin were assessed. The efficacy of CNDS@Simvastatin in improving keloids and the biocompatibility and safety of CNDS@Simvastatin were examined in vivo by establishing a murine ear keloid model.
Results: CNDS@Simvastatin showed sustained and uniform inhibition of the proliferation of fibroblasts, Col Ι, and CTGF via the gradual release of simvastatin over 72 h. It was efficient in the treatment of the murine ear keloid with no observable toxic side effects on various organs.
Conclusion: Simvastatin-loaded copolymer acid-sensitive nanocarriers, CNDS@Simvastatin nanospheres, were successfully developed in this study, and these were characterized by favorable physicochemical properties and biocompatibility.
Keywords: PEG; PH; PLGA; complex nanocontrolled delivery system; fibroblasts; keloid; simvastatin.
© 2024 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Mun J. H., Kim Y. M., Kim B. S., Kim J. H., Kim M. B., and Ko H. C., “Simvastatin Inhibits Transforming Growth Factor‐β1‐Induced Expression of Type I Collagen, CTGF, and α‐SMA in Keloid Fibroblasts,” Wound Repair and Regeneration 22, no. 1 (2014): 125–133. - PubMed
-
- Hietanen K. E., Järvinen T. A., Huhtala H., Tolonen T. T., Kuokkanen H. O., and Kaartinen I. S., “Treatment of Keloid Scars With Intralesional Triamcinolone and 5‐Fluorouracil Injections—A Randomized Controlled Trial,” Journal of Plastic, Reconstructive & Aesthetic Surgery 72, no. 1 (2019): 4–11. - PubMed
-
- Yan Q., Xiao L. Q., Tan L., et al., “Controlled Release of Simvastatin‐Loaded Thermo‐Sensitive PLGA‐PEG‐PLGA Hydrogel for Bone Tissue Regeneration: In Vitro and In Vivo Characteristics,” Journal of Biomedical Materials Research. Part A 103, no. 11 (2015): 3580–3589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

