WTAP-mediated m6A modification of TRIM22 promotes diabetic nephropathy by inducing mitochondrial dysfunction via ubiquitination of OPA1
- PMID: 39314036
- PMCID: PMC11423538
- DOI: 10.1080/13510002.2024.2404794
WTAP-mediated m6A modification of TRIM22 promotes diabetic nephropathy by inducing mitochondrial dysfunction via ubiquitination of OPA1
Abstract
Objectives: Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and is the most common cause of end-stage renal disease. Tripartite motif-containing (TRIM) proteins are a large family of E3 ubiquitin ligases that contribute to protein quality control by regulating the ubiquitin - proteasome system. However, the detailed mechanisms through which various TRIM proteins regulate downstream events have not yet been fully elucidated. The current research aimed to determine the function and mechanism of TRIM22 in DN.
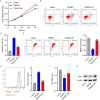
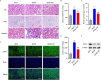
Methods: DN models were established by inducing HK-2 cells using high glucose (HG) and diabetic mice (db/db mice). Cell viability, apoptosis, mitochondrial reactive oxygen species, and mitochondrial membrane potential were detected by Cell Counting Kit-8 and flow cytometry, respectively. Pathological changes were evaluated using hematoxylin and eosin, periodic acid schiff and Masson staining. The binding between TRIM22 and optic atrophy 1 (OPA1) was analyzed using co-immunoprecipitation. The m6A level of TRIM22 5'UTR was detected using RNA immunoprecipitation.
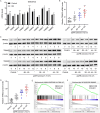
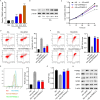
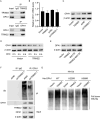
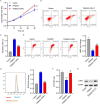
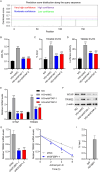
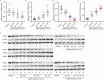
Results: TRIM22 was highly expressed in patients with DN. TRIM22 silencing inhibited HG-induced apoptosis and mitochondrial dysfunction in HK-2 cells. Promoting mitochondrial fusion alleviated TRIM22 overexpression-induced cell apoptosis, mitochondrial dysfunction in HK-2 cells, and kidney damage in mice. Mechanistically, TRIM22 interacted with OPA1 and induced its ubiquitination. Wilms tumor 1-associating protein (WTAP) promoted m6A modification of TRIM22 through the m6A reader insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1).
Discussion: TRIM22 silencing inhibited the progression of DN by interacting with OPA1 and inducing its ubiquitination. Furthermore, WTAP promoted m6A modification of TRIM22 via IGF2BP1.
Keywords: OPA1; TRIM22; WTAP; diabetic nephropathy; m6A; mitochondrial dysfunction.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
