Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial
- PMID: 39314133
- PMCID: PMC11791568
- DOI: 10.3350/cmh.2024.0629
Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial
Abstract
Background/aims: A clinical unmet need persists for medications capable of modulating the progression of primary sclerosing cholangitis (PSC). This study aimed to assess the clinical feasibility of HK-660S (beta-lapachone) in PSC.
Methods: In this multicenter, randomized, double-blind, placebo-controlled, parallel-group phase 2 trial, participants were assigned in a 2:1 ratio to receive either 100 mg of HK-660S or a placebo twice daily for 12 weeks. The primary outcomes were the reduction in serum alkaline phosphatase (ALP) levels and the percentage of participants showing improvements in PSC severity, as determined by magnetic resonance cholangiopancreatography with the Anali score. Secondary endpoints included changes in liver stiffness and adverse events.
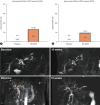
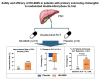
Results: The analysis included 21 patients, 15 receiving HK-660S, and six receiving a placebo. Improvements in the Anali score were observed in 13.3% of the HK-660S group, with no improvements in the placebo group. HK-660S treatment resulted in a 15.2% reduction in mean ALP levels, compared to a 6.6% reduction in the placebo group. A stratified ad-hoc analysis based on baseline ALP levels showed a statistically significant response in the HK-660S group among those with ALP levels greater than twice the upper limit of normal, with a 50% responder rate (p=0.05). Additionally, 26.7% of the HK-660S group showed improvements in the enhanced liver fibrosis score, with no improvements in the placebo group. HK-660S was generally well tolerated.
Conclusion: HK-660S is well tolerated among patients with PSC and may improve bile duct strictures, decrease serum ALP levels, and reduce liver fibrosis (cris.nih.go.kr, Number KCT0006590).
Keywords: Alkaline phosphatase; Cholangiopancreatography, magnetic resonance; Lapachone; Nicotinamide adenine dinucleotide; Primary sclerosing cholangitis.
Conflict of interest statement
Heon Se Jeong and Hee Jin Kim are Curome Biosciences Co., Ltd employees. The other authors disclose no conflicts.
Figures


References
-
- Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–1323. - PubMed
-
- Trauner M, Bowlus CL, Gulamhusein A, Hameed B, Caldwell SH, Shiffman ML, et al. Safety and sustained efficacy of the farnesoid X receptor (FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC. Clin Gastroenterol Hepatol. 2023;21:1552–1560.e2. - PubMed
-
- Trauner M, Levy C, Tanaka A, Goodman Z, Thorburn D, Joshi D, et al. A phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of cilofexor in patients with non-cirrhotic primary sclerosing cholangitis (PRIMIS) J Hepatol. 2023;78(Supplement 1):S12–S13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

