Nanocarrier-mediated siRNA delivery: a new approach for the treatment of traumatic brain injury-related Alzheimer's disease
- PMID: 39314170
- PMCID: PMC11801294
- DOI: 10.4103/NRR.NRR-D-24-00303
Nanocarrier-mediated siRNA delivery: a new approach for the treatment of traumatic brain injury-related Alzheimer's disease
Abstract
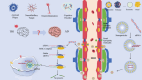
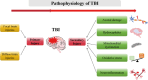
Traumatic brain injury and Alzheimer's disease share pathological similarities, including neuronal loss, amyloid-β deposition, tau hyperphosphorylation, blood-brain barrier dysfunction, neuroinflammation, and cognitive deficits. Furthermore, traumatic brain injury can exacerbate Alzheimer's disease-like pathologies, potentially leading to the development of Alzheimer's disease. Nanocarriers offer a potential solution by facilitating the delivery of small interfering RNAs across the blood-brain barrier for the targeted silencing of key pathological genes implicated in traumatic brain injury and Alzheimer's disease. Unlike traditional approaches to neuroregeneration, this is a molecular-targeted strategy, thus avoiding non-specific drug actions. This review focuses on the use of nanocarrier systems for the efficient and precise delivery of siRNAs, discussing the advantages, challenges, and future directions. In principle, siRNAs have the potential to target all genes and non-targetable proteins, holding significant promise for treating various diseases. Among the various therapeutic approaches currently available for neurological diseases, siRNA gene silencing can precisely "turn off" the expression of any gene at the genetic level, thus radically inhibiting disease progression; however, a significant challenge lies in delivering siRNAs across the blood-brain barrier. Nanoparticles have received increasing attention as an innovative drug delivery tool for the treatment of brain diseases. They are considered a potential therapeutic strategy with the advantages of being able to cross the blood-brain barrier, targeted drug delivery, enhanced drug stability, and multifunctional therapy. The use of nanoparticles to deliver specific modified siRNAs to the injured brain is gradually being recognized as a feasible and effective approach. Although this strategy is still in the preclinical exploration stage, it is expected to achieve clinical translation in the future, creating a new field of molecular targeted therapy and precision medicine for the treatment of Alzheimer's disease associated with traumatic brain injury.
Copyright © 2025 Copyright: © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures
Similar articles
-
Nanocarrier-based targeted drug delivery for Alzheimer's disease: addressing neuroinflammation and enhancing clinical translation.Front Pharmacol. 2025 May 14;16:1591438. doi: 10.3389/fphar.2025.1591438. eCollection 2025. Front Pharmacol. 2025. PMID: 40438598 Free PMC article. Review.
-
Liposome based drug delivery as a potential treatment option for Alzheimer's disease.Neural Regen Res. 2022 Jun;17(6):1190-1198. doi: 10.4103/1673-5374.327328. Neural Regen Res. 2022. PMID: 34782553 Free PMC article. Review.
-
Systemic peptide mediated delivery of an siRNA targeting α-syn in the CNS ameliorates the neurodegenerative process in a transgenic model of Lewy body disease.Neurobiol Dis. 2019 Jul;127:163-177. doi: 10.1016/j.nbd.2019.03.001. Epub 2019 Mar 5. Neurobiol Dis. 2019. PMID: 30849508 Free PMC article.
-
Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer's disease.Neural Regen Res. 2023 Oct;18(10):2127-2133. doi: 10.4103/1673-5374.369096. Neural Regen Res. 2023. PMID: 37056119 Free PMC article. Review.
-
Focused ultrasound therapy for Alzheimer's disease: exploring the potential for targeted amyloid disaggregation.Front Neurol. 2024 Aug 6;15:1426075. doi: 10.3389/fneur.2024.1426075. eCollection 2024. Front Neurol. 2024. PMID: 39165269 Free PMC article.
Cited by
-
Functional Verification of Differentially Expressed Genes Following DENV2 Infection in Aedes aegypti.Viruses. 2025 Jan 6;17(1):67. doi: 10.3390/v17010067. Viruses. 2025. PMID: 39861856 Free PMC article.
-
Traumatic brain injury: Bridging pathophysiological insights and precision treatment strategies.Neural Regen Res. 2026 Mar 1;21(3):887-907. doi: 10.4103/NRR.NRR-D-24-01398. Epub 2025 Mar 25. Neural Regen Res. 2026. PMID: 40145994 Free PMC article.
-
hnRNPH1: A Multifaceted Regulator in RNA Processing and Disease Pathogenesis.Int J Mol Sci. 2025 May 28;26(11):5159. doi: 10.3390/ijms26115159. Int J Mol Sci. 2025. PMID: 40507967 Free PMC article. Review.
References
-
- Abrahamson EE, Ikonomovic MD. Brain injury-induced dysfunction of the blood brain barrier as a risk for dementia. Exp Neurol. 2020;328:113257. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

