Validation of a PD-1/CD28 chimeric switch receptor to augment CAR-T function in dogs with spontaneous B cell lymphoma
- PMID: 39314237
- PMCID: PMC11418608
- DOI: 10.1016/j.isci.2024.110863
Validation of a PD-1/CD28 chimeric switch receptor to augment CAR-T function in dogs with spontaneous B cell lymphoma
Abstract
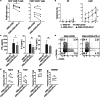
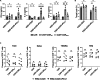
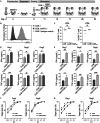
Chimeric antigen receptor (CAR) T cell therapy has achieved unprecedented clinical outcomes in patients with relapsed/refractory B cell leukemias; however, response rates in patients with large B cell lymphoma (LBCL) are less impressive. Expression of PD-1 on activated T cells and PD-L1 on malignant, stromal, and immune cells within the tumor microenvironment (TME) contribute to CAR-T exhaustion, hypofunction, and treatment failures. Here, a comparative approach is taken to develop a chimeric switch receptor (CSR) with potential to augment CAR-T persistence, function, and clinical efficacy in immune competent, pet dogs with spontaneous B cell lymphoma (BCL). We show that similar to human CAR-T cells, expression of a PD-1/CD28 CSR in canine CAR-T cells results in enhanced function against PD-L1+ targets and preserves central memory phenotype. We also demonstrate that these effects depend upon active CSR signaling. This work paves the way for in vivo studies in canine BCL patients to inform human trial design.
Keywords: Cancer; Therapeutics; Veterinary medicine.
© 2024 The Author(s).
Conflict of interest statement
N.J.M is a co-founder of Vetigenics.
Figures



References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
-
- Shah B.D., Ghobadi A., Oluwole O.O., Logan A.C., Boissel N., Cassaday R.D., Leguay T., Bishop M.R., Topp M.S., Tzachanis D., et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. doi: 10.1016/S0140-6736(21)01222-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

