β-hydroxybutyrate recapitulates the beneficial effects of ketogenic metabolic therapy in polycystic kidney disease
- PMID: 39314240
- PMCID: PMC11418134
- DOI: 10.1016/j.isci.2024.110773
β-hydroxybutyrate recapitulates the beneficial effects of ketogenic metabolic therapy in polycystic kidney disease
Abstract
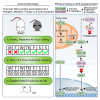
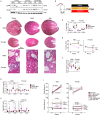
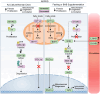
Autosomal-dominant polycystic kidney disease (ADPKD) is a common monogenic disease characterized by the formation of fluid-filled renal cysts, loss of mitochondrial function, decreased fatty acid oxidation, increased glycolysis, and likely renal failure. We previously demonstrated that inducing a state of ketosis ameliorates or reverses PKD progression in multiple animal models. In this study, we compare time-restricted feeding and 48-h periodic fasting regimens in both juvenile and adult Cy/+ rats. Both fasting regimens potently prevent juvenile disease progression and partially reverse PKD in adults. To explore the mechanism of fasting, we administered β-hydroxybutyrate (BHB) to Cy/+ rats and orthologous mouse models of PKD (Pkd1 RC/RC , Pkd1-Ksp:Cre). BHB recapitulated the effects of fasting in these models independent of stereoisomer, suggesting the effects of BHB are largely due to its signaling functions. These findings implicate the use of ketogenic metabolic therapy and BHB supplementation as potential disease modifiers of PKD and point toward underlying mechanisms.
Keywords: Diet; Pathophysiology; Therapy.
© 2024 The Author(s).
Conflict of interest statement
J.A.T. and T.W. are partial owners in the Benefit Corporation Santa Barbara Nutrients and are inventors on US patent No. 11,013,705 and International Publication No. WO 2020/186154 A1 for the use of the combination of BHB and citrate in PKD. T.W. was on the scientific advisory board of Chinook Therapeutics and has received research funding from Chinook Therapeutics and Kyowa Kirin, and speaker fees from Otsuka. T.W. was on the scientific advisory board of Chinook Therapeutics.
Figures






References
-
- Rossetti S., Consugar M.B., Chapman A.B., Torres V.E., Guay-Woodford L.M., Grantham J.J., Bennett W.M., Meyers C.M., Walker D.L., Bae K., et al. Comprehensive Molecular Diagnostics in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. - DOI - PubMed

