CYP2C19 genotype and sodium channel blockers in lacosamide-treated children with epilepsy: two major determinants of trough lacosamide concentration or clinical response
- PMID: 39314259
- PMCID: PMC11418302
- DOI: 10.1177/17562864241273087
CYP2C19 genotype and sodium channel blockers in lacosamide-treated children with epilepsy: two major determinants of trough lacosamide concentration or clinical response
Abstract
Background: The widespread clinical use of lacosamide (LCM) has revealed significant individual differences in clinical response, with various reported influencing factors. However, it remains unclear how genetic factors related to the disposition and clinical response of LCM, as well as drug-drug interactions (DDIs), exert their influence on pediatric patients with epilepsy.
Objectives: To evaluate the impact of genetic variations and DDIs on plasma LCM concentrations and clinical response.
Design: Patients with epilepsy treated with LCM from June 2021 to March 2023 in the Children's Hospital of Nanjing Medical University were included in the analysis.
Methods: The demographic information and laboratory examination data were obtained from the hospital information system. For the pharmacogenetic study, the left-over blood specimens, collected for routine plasma LCM concentration monitoring, were used to perform genotyping analysis for the selected 26 single nucleotide polymorphisms from 14 genes. The trough concentration/daily dose (C 0/D) ratio and efficacy outcomes were compared.
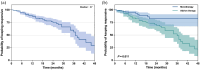
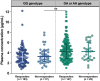
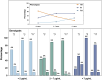
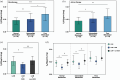
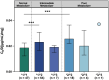
Results: Patients achieved 90.1% and 68.9% responder rates in LCM mono- and add-on therapy, respectively. The genetic variant in the CYP2C19 *2 (rs4244285) was associated with a better responsive treatment outcome (odds ratio: 1.82; 95% confidence interval: 1.05-3.15; p = 0.031). In monotherapy, 36% of patients were CYP2C19 normal metabolizers (NMs), 49% were intermediate metabolizers (IMs), and 15% were poor metabolizers (PMs) carrying CYP2C19 *2 or *3. Of note, the C 0/D ratios of IMs and PMs were 9.1% and 39.6% higher than those of NMs, respectively. Similar results were in the add-on therapy group, and we also observed a substantial decrease in the C 0/D ratio when patients were concomitant with sodium channel blockers (SCBs).
Conclusion: This study was the first to confirm that CYP2C19 *2 or *3 variants impact the disposition and treatment response of LCM in children with epilepsy. Moreover, concomitant with SCBs, particularly oxcarbazepine, also decreased plasma LCM concentration.
Keywords: CYP2C19; children; focal epilepsy; lacosamide; therapeutic drug monitoring.
Plain language summary
CYP2C19 genotype and sodium channel blockers in lacosamide-treated children with epilepsy: two major determinants of plasma lacosamide concentration or treatment efficacy This study examined the impact of genetic factors and drug combinations on the effectiveness and plasma concentrations of lacosamide, an antiseizure medication, in patients under 18. Analyzing blood samples from 316 patients at the Children’s Hospital of Nanjing Medical University, researchers discovered that genetic variations in the CYP2C19 (i.e. *2 and *3), along with metabolic capacity, and co-medication with sodium channel blockers, all influence plasma lacosamide concentration. Understanding these genetic influences could inform personalized dosing strategies, improving the medication’s management for pediatric epilepsy patients.
© The Author(s), 2024.
Figures


Similar articles
-
Effects of CYP2C19 and CYP2C9 polymorphisms on the efficacy and plasma concentration of lacosamide in pediatric patients with epilepsy in China.Eur J Pediatr. 2024 Dec 10;184(1):73. doi: 10.1007/s00431-024-05897-6. Eur J Pediatr. 2024. PMID: 39658609
-
Effects of CYP2C19 genetic polymorphisms on the pharmacokinetics of lacosamide in Korean patients with epilepsy.Epilepsia. 2022 Nov;63(11):2958-2969. doi: 10.1111/epi.17399. Epub 2022 Aug 30. Epilepsia. 2022. PMID: 36039802
-
Plasma lacosamide monitoring in children with epilepsy: Focus on reference therapeutic range and influencing factors.Front Pediatr. 2022 Sep 7;10:949783. doi: 10.3389/fped.2022.949783. eCollection 2022. Front Pediatr. 2022. PMID: 36160782 Free PMC article.
-
Lacosamide Therapy and CYP2C19 Genotype.2018 Apr 18. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. 2018 Apr 18. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. PMID: 29671994 Free Books & Documents. Review.
-
A common reference-based indirect comparison meta-analysis of eslicarbazepine versus lacosamide as add on treatments for focal epilepsy.Epilepsy Res. 2016 Nov;127:12-18. doi: 10.1016/j.eplepsyres.2016.08.006. Epub 2016 Aug 11. Epilepsy Res. 2016. PMID: 27543806 Review.
References
-
- Auvin S, Arzimanoglou A, Beller C, et al.. Safety, tolerability, and efficacy of adjunctive lacosamide in pediatric patients with epilepsy syndromes associated with generalized seizures: phase 2, open-label exploratory trial. Epilepsia 2023; 64: 2947–2957. - PubMed
-
- Rosati A, Ilvento L, Rizzi R, et al.. Long-term efficacy of add-on lacosamide treatment in children and adolescents with refractory epilepsies: a single-center observational study. Epilepsia 2018; 59: 1004–1010. - PubMed
-
- Cawello W, Boekens H, Bonn R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: mass balance following intravenous and oral administration. Eur J Drug Metab Pharmacokinet 2012; 37: 241–248. - PubMed
LinkOut - more resources
Full Text Sources

