This is a preprint.
ATP-dependent remodeling of chromatin condensates uncovers distinct mesoscale effects of two remodelers
- PMID: 39314305
- PMCID: PMC11418981
- DOI: 10.1101/2024.09.10.611504
ATP-dependent remodeling of chromatin condensates uncovers distinct mesoscale effects of two remodelers
Update in
-
ATP-dependent remodeling of chromatin condensates reveals distinct mesoscale outcomes.Science. 2025 Oct 2;390(6768):eadr0018. doi: 10.1126/science.adr0018. Epub 2025 Oct 2. Science. 2025. PMID: 41037607
Abstract
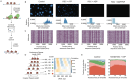
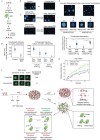
ATP-dependent chromatin remodeling enzymes mobilize nucleosomes, but how such mobilization affects chromatin condensation is unclear. Here, we investigate effects of two major remodelers, ACF and RSC using chromatin condensates and single-molecule footprinting. We find that both remodelers inhibit the formation of condensed chromatin. However, the remodelers have distinct effects on pre-formed chromatin condensates. ACF spaces nucleosomes without de-condensing the chromatin, explaining how ACF maintains nucleosome organization in transcriptionally repressed genomic regions. In contrast, RSC catalyzes ATP-dependent de-condensation of chromatin. Surprisingly, RSC also drives micron-scale movements of entire condensates. These newly uncovered activities of RSC explain its central role in transcriptional activation. The biological importance of remodelers may thus reflect both their effects on nucleosome mobilization and the corresponding consequences on chromatin dynamics at the mesoscale.
Conflict of interest statement
Competing interests: G.J.N is a co-founder of TippingPoint Biosciences.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
