This is a preprint.
The Structure of Cilium Inner Junctions Revealed by Electron Cryo-tomography
- PMID: 39314311
- PMCID: PMC11419100
- DOI: 10.1101/2024.09.09.612100
The Structure of Cilium Inner Junctions Revealed by Electron Cryo-tomography
Update in
-
The structure of basal body inner junctions from Tetrahymena revealed by electron cryo-tomography.EMBO J. 2025 Apr;44(7):1975-2001. doi: 10.1038/s44318-025-00392-6. Epub 2025 Feb 24. EMBO J. 2025. PMID: 39994484 Free PMC article.
Abstract
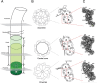
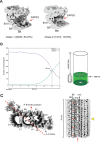
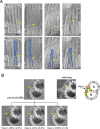
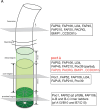
The cilium is a microtubule-based organelle critical for many cellular functions. Its assembly initiates at a basal body and continues as an axoneme that projects out of the cell to form a functional cilium. This assembly process is tightly regulated. However, our knowledge of the molecular architecture and the mechanism of assembly is limited. By applying electron cryotomography and subtomogram averaging, we obtained subnanometer resolution structures of the inner junction in three distinct regions of the cilium: the proximal region of the basal body, the central core of the basal body, and the flagellar axoneme. The structures allowed us to identify several basal body and axoneme components. While a few proteins are distributed throughout the entire length of the organelle, many are restricted to particular regions of the cilium, forming intricate local interaction networks and bolstering local structural stability. Finally, by knocking out a critical basal body inner junction component Poc1, we found the triplet MT was destabilized, resulting in a defective structure. Surprisingly, several axoneme-specific components were found to "infiltrate" into the mutant basal body. Our findings provide molecular insight into cilium assembly at its inner Junctions, underscoring its precise spatial regulation.
Keywords: assembly; basal body; centriole; cilium; electron cryo-tomography.
Figures









Similar articles
-
The structure of basal body inner junctions from Tetrahymena revealed by electron cryo-tomography.EMBO J. 2025 Apr;44(7):1975-2001. doi: 10.1038/s44318-025-00392-6. Epub 2025 Feb 24. EMBO J. 2025. PMID: 39994484 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Abramson J., Adler J., Dunger J., Evans R., Green T., Pritzel A., Ronneberger O., Willmore L., Ballard A.J., Bambrick J., Bodenstein S.W., Evans D.A., Hung C.-C., O’Neill M., Reiman D., Tunyasuvunakool K., Wu Z., Žemgulytė A., Arvaniti E., Beattie C., Bertolli O., Bridgland A., Cherepanov A., Congreve M., Cowen-Rivers A.I., Cowie A., Figurnov M., Fuchs F.B., Gladman H., Jain R., Khan Y.A., Low C.M.R., Perlin K., Potapenko A., Savy P., Singh S., Stecula A., Thillaisundaram A., Tong C., Yakneen S., Zhong E.D., Zielinski M., Žídek A., Bapst V., Kohli P., Jaderberg M., Hassabis D., and Jumper J.M.. 2024. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 630:493–500. doi:10.1038/s41586-024-07487-w. - DOI - PMC - PubMed
-
- Breugel M. van, Hirono M., Andreeva A., Yanagisawa H., Yamaguchi S., Nakazawa Y., Morgner N., Petrovich M., Ebong I.-O., Robinson C.V., Johnson C.M., Veprintsev D., and Zuber B.. 2011. Structures of SAS-6 Suggest Its Organization in Centrioles. Science. 331:1196–1199. doi:10.1126/science.1199325. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
