This is a preprint.
The myokine FGF21 associates with enhanced survival in ALS and mitigates stress-induced cytotoxicity
- PMID: 39314333
- PMCID: PMC11419072
- DOI: 10.1101/2024.09.11.611693
The myokine FGF21 associates with enhanced survival in ALS and mitigates stress-induced cytotoxicity
Update in
-
The myokine FGF21 associates with enhanced survival in ALS and mitigates stress-induced cytotoxicity.Aging (Albany NY). 2025 Aug 9;17(8):2033-2062. doi: 10.18632/aging.206298. Epub 2025 Aug 9. Aging (Albany NY). 2025. PMID: 40788112 Free PMC article.
Abstract
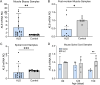
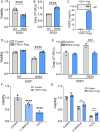
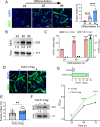
Amyotrophic lateral sclerosis (ALS) is an age-related and fatal neurodegenerative disease characterized by progressive muscle weakness. There is marked heterogeneity in clinical presentation, progression, and pathophysiology with only modest treatments to slow disease progression. Molecular markers that provide insight into this heterogeneity are crucial for clinical management and identification of new therapeutic targets. In a prior muscle miRNA sequencing investigation, we identified altered FGF pathways in ALS muscle, leading us to investigate FGF21. We analyzed human ALS muscle biopsy samples and found a large increase in FGF21 expression with localization to atrophic myofibers and surrounding endomysium. A concomitant increase in FGF21 was detected in ALS spinal cords which correlated with muscle levels. FGF21 was increased in the SOD1G93A mouse beginning in presymptomatic stages. In parallel, there was dysregulation of the co-receptor, β-Klotho. Plasma FGF21 levels were increased and high levels correlated with slower disease progression, prolonged survival, and increased body mass index. In NSC-34 motor neurons and C2C12 muscle cells expressing SOD1G93A or exposed to oxidative stress, ectopic FGF21 mitigated loss of cell viability. In summary, FGF21 is a novel biomarker in ALS that correlates with slower disease progression and exerts trophic effects under conditions of cellular stress.
Keywords: ALS biomarker; Fibroblast growth factor 21; human skeletal muscle; motor neurons; oxidative stress response; β-Klotho.
Conflict of interest statement
Conflicts of Interest The authors report no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
