This is a preprint.
Death in the taste bud: engulfment of dying taste receptor cells by glial-like Type I cells
- PMID: 39314340
- PMCID: PMC11418956
- DOI: 10.1101/2024.09.06.611711
Death in the taste bud: engulfment of dying taste receptor cells by glial-like Type I cells
Update in
-
Death in the Taste Bud: Engulfment of Dying Taste Receptor Cells by Glial-Like Type I Cells.Glia. 2025 Aug;73(8):1660-1672. doi: 10.1002/glia.70025. Epub 2025 Apr 30. Glia. 2025. PMID: 40304099
Abstract
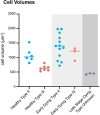
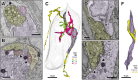
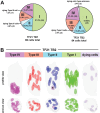
Taste buds comprise 50-100 epithelial derived cells, including glial-like cells (Type I) and two types of receptor cells (Types II and III). All of these taste cells are renewed throughout the life of an organism from a pool of uncommitted basal cells. Immature cells enter the bud at its base, maturing into one of the three mature cell types. How taste cells die and/or exit the bud, as well as the role of the glial-like cells in this process, remains unclear. Here we present morphological data obtained through Serial Blockface Scanning Electron Microscopy of murine circumvallate taste buds, revealing taste cells at the end of their life. Cells we identify as dying share morphological features typical of apoptosis: swollen endoplasmic reticulum, large lysosomes, degrading organelles, distended outer nuclear membranes, heterochromatin reorganization, cell shrinkage, and cell and/or nuclear fragmentation. Most early stage dying cells have Type II cell morphologies, while a few display Type III cell features. Many dying cells maintain contacts with nerve fibers, but those postsynaptic fibers often appear to be detached from the main trunk of an afferent nerve. Dying cells, like mature Type II and III taste cells, are surrounded by glial-like Type I cells. In many instances Type I cells appear to be engulfing their dying neighbors, suggesting for them a novel, phagocytic role. Surprisingly, virtually no Type I cells display features of apoptosis, although they reportedly have the shortest residence time in taste buds. The ultimate fate of Type I cells therefore remains unknown.
Keywords: apoptosis; cell death; electron microscopy; engulfment; glial-like; synapse; taste buds.
Figures






Similar articles
-
Death in the Taste Bud: Engulfment of Dying Taste Receptor Cells by Glial-Like Type I Cells.Glia. 2025 Aug;73(8):1660-1672. doi: 10.1002/glia.70025. Epub 2025 Apr 30. Glia. 2025. PMID: 40304099
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Survivor, family and professional experiences of psychosocial interventions for sexual abuse and violence: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2022 Oct 4;10(10):CD013648. doi: 10.1002/14651858.CD013648.pub2. Cochrane Database Syst Rev. 2022. PMID: 36194890 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Araque A., Parpura V., Sanzgiri R. P., & Haydon P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci, 22(5), 208–215. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
