This is a preprint.
Impact of anatomic variability and other vascular factors on lamina cribrosa hypoxia
- PMID: 39314360
- PMCID: PMC11419109
- DOI: 10.1101/2024.09.12.610282
Impact of anatomic variability and other vascular factors on lamina cribrosa hypoxia
Abstract
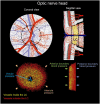
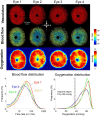
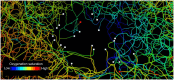
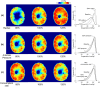
Insufficient oxygenation in the lamina cribrosa (LC) may contribute to axonal damage and glaucomatous vision loss. To understand the range of susceptibilities to glaucoma, we aimed to identify key factors influencing LC oxygenation and examine if these factors vary with anatomical differences between eyes. We reconstructed 3D, eye-specific LC vessel networks from histological sections of four healthy monkey eyes. For each network, we generated 125 models varying vessel radius, oxygen consumption rate, and arteriole perfusion pressure. Using hemodynamic and oxygen supply modeling, we predicted blood flow distribution and tissue oxygenation in the LC. ANOVA assessed the significance of each parameter. Our results showed that vessel radius had the greatest influence on LC oxygenation, followed by anatomical variations. Arteriole perfusion pressure and oxygen consumption rate were the third and fourth most influential factors, respectively. The LC regions are well perfused under baseline conditions. These findings highlight the importance of vessel radius and anatomical variation in LC oxygenation, providing insights into LC physiology and pathology. Pathologies affecting vessel radius may increase the risk of LC hypoxia, and anatomical variations could influence susceptibility. Conversely, increased oxygen consumption rates had minimal effects, suggesting that higher metabolic demands, such as those needed to maintain intracellular transport despite elevated intraocular pressure, have limited impact on LC oxygenation.
Keywords: blood flow; glaucoma; hemodynamics; lamina cribrosa; oxygenation; vasculature.
Conflict of interest statement
Conflict of Interest: none
Figures

Similar articles
-
Lamina Cribrosa Hypoxia Sensitivity to Variations of Anatomy and Vascular Factors.J Biomech Eng. 2025 Aug 1;147(8):081002. doi: 10.1115/1.4068577. J Biomech Eng. 2025. PMID: 40314737
-
Eye-specific 3D modeling of factors influencing oxygen concentration in the lamina cribrosa.Exp Eye Res. 2022 Jul;220:109105. doi: 10.1016/j.exer.2022.109105. Epub 2022 May 12. Exp Eye Res. 2022. PMID: 35568202 Free PMC article.
-
Impact of elevated IOP on lamina cribrosa oxygenation; A combined experimental-computational study on monkeys.bioRxiv [Preprint]. 2024 Sep 10:2024.09.05.609208. doi: 10.1101/2024.09.05.609208. bioRxiv. 2024. Update in: Ophthalmol Sci. 2025 Jan 31;5(3):100725. doi: 10.1016/j.xops.2025.100725. PMID: 39314421 Free PMC article. Updated. Preprint.
-
The interaction between intracranial pressure, intraocular pressure and lamina cribrosal compression in glaucoma.Clin Exp Optom. 2016 May;99(3):219-26. doi: 10.1111/cxo.12333. Epub 2016 Apr 15. Clin Exp Optom. 2016. PMID: 27079432 Review.
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
References
-
- Quigley H., and Anderson D. R., 1976, "The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve," Investigative ophthalmology & visual science, 15(8), pp. 606–616. - PubMed
-
- Quigley H. A., Nickells R. W., Kerrigan L. A., Pease M. E., Thibault D. J., and Zack D. J., 1995, "Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis," Investigative ophthalmology & visual science, 36(5), pp. 774–786. - PubMed
-
- Howell G. R., Libby R. T., Jakobs T. C., Smith R. S., Phalan F. C., Barter J. W., Barbay J. M., Marchant J. K., Mahesh N., and Porciatti V., 2007, "Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma," The Journal of cell biology, 179(7), pp. 1523–1537. - PMC - PubMed
-
- Quigley H., 2011, "vol. 377," Glaucoma Lancet, pp. 1367–1377. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
