This is a preprint.
Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity
- PMID: 39314375
- PMCID: PMC11419099
- DOI: 10.1101/2024.09.09.612138
Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity
Update in
-
Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity.Cell Rep. 2025 Jul 22;44(7):115954. doi: 10.1016/j.celrep.2025.115954. Epub 2025 Jul 9. Cell Rep. 2025. PMID: 40638386 Free PMC article.
Abstract
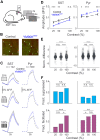
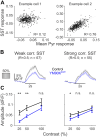
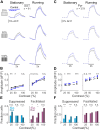
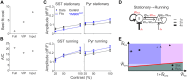
Inhibition stabilization enables cortical circuits to encode sensory signals across diverse contexts. Somatostatin-expressing (SST) interneurons are well-suited for this role through their strong recurrent connectivity with excitatory pyramidal cells. We developed a cortical circuit model predicting that SST cells become increasingly important for stabilization as sensory input strengthens. We tested this prediction in mouse primary visual cortex by manipulating excitatory input to SST cells, a key parameter for inhibition stabilization, with a novel cell-type specific pharmacological method to selectively block glutamatergic receptors on SST cells. Consistent with our model predictions, we find antagonizing glutamatergic receptors drives a paradoxical facilitation of SST cells with increasing stimulus contrast. In addition, we find even stronger engagement of SST-dependent stabilization when the mice are aroused. Thus, we reveal that the role of SST cells in cortical processing gradually switches as a function of both input strength and behavioral state.
Keywords: AMPA receptors; calcium imaging; chemogenetics; inhibition; microcircuit; normalization; pharmacology; visual cortex.
Conflict of interest statement
Declaration of Interests M.R.T. and B.C.S are on a patent application describing HTL.2 and its applications. The remaining authors declare no competing interests.
Figures


References
-
- Heeger D.J. (1992). Normalization of cell responses in cat striate cortex. Vis. Neurosci. 9, 181–197. - PubMed
-
- Palmigiano A., Fumarola F., Mossing D.P., Kraynyukova N., Adesnik H., and Miller K.D. (2023). Common rules underlying optogenetic and behavioral modulation of responses in multi-cell-type V1 circuits. bioRxiv, 2020.11.11.378729. 10.1101/2020.11.11.378729. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
