This is a preprint.
NR2F2 Reactivation in Early-life Adipocyte Stem-like Cells Rescues Adipocyte Mitochondrial Oxidation
- PMID: 39314382
- PMCID: PMC11419096
- DOI: 10.1101/2024.09.09.611047
NR2F2 Reactivation in Early-life Adipocyte Stem-like Cells Rescues Adipocyte Mitochondrial Oxidation
Abstract
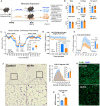
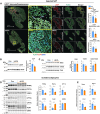
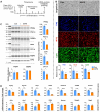
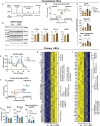
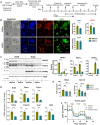
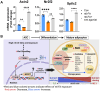
In humans, perinatal exposure to an elevated omega-6 (n6) relative to omega-3 (n3) Fatty Acid (FA) ratio is associated with the likelihood of childhood obesity. In mice, we show perinatal exposure to excessive n6-FA programs neonatal Adipocyte Stem-like cells (ASCs) to differentiate into adipocytes with lower mitochondrial nutrient oxidation and a propensity for nutrient storage. Omega-6 FA exposure reduced fatty acid oxidation (FAO) capacity, coinciding with impaired induction of beige adipocyte regulatory factors PPARγ, PGC1α, PRDM16, and UCP1. ASCs from n6-FA exposed pups formed adipocytes with increased lipogenic genes in vitro, consistent with an in vivo accelerated adipocyte hypertrophy, greater triacylglyceride accumulation, and increased % body fat. Conversely, n6-FA exposed pups had impaired whole animal 13C-palmitate oxidation. The metabolic nuclear receptor, NR2F2, was suppressed in ASCs by excess n6-FA intake preceding adipogenesis. ASC deletion of NR2F2, prior to adipogenesis, mimicked the reduced FAO capacity observed in ASCs from n6-FA exposed pups, suggesting that NR2F2 is required in ASCs for robust beige regulator expression and downstream nutrient oxidation in adipocytes. Transiently re-activating NR2F2 with ligand prior to differentiation in ASCs from n6-FA exposed pups, restored their FAO capacity as adipocytes by increasing the PPARγ-PGC1α axis, mitochondrial FA transporter CPT1A, ATP5 family synthases, and NDUF family Complex I proteins. Our findings suggest that excessive n6-FA exposure early in life dampens an NR2F2-mediated induction of beige adipocyte regulators, resulting in metabolic programming that is shifted towards nutrient storage.
Keywords: NR2F2; adipocyte stem-like cells; beige adipocyte metabolism; early-life adipocyte development; omega-3 fatty acids.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures

Similar articles
-
Activating nuclear receptor subfamily 2 group F member 2 in adipocyte stem cells rescues beige adipocyte metabolism impaired by excess early-life omega-6 fatty acids.Clin Nutr. 2025 Aug;51:63-80. doi: 10.1016/j.clnu.2025.06.003. Epub 2025 Jun 10. Clin Nutr. 2025. PMID: 40527120
-
Neonatal intake of Omega-3 fatty acids enhances lipid oxidation in adipocyte precursors.iScience. 2022 Dec 6;26(1):105750. doi: 10.1016/j.isci.2022.105750. eCollection 2023 Jan 20. iScience. 2022. PMID: 36590177 Free PMC article.
-
Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming.Clin Epigenetics. 2020 Nov 23;12(1):181. doi: 10.1186/s13148-020-00970-x. Clin Epigenetics. 2020. PMID: 33228780 Free PMC article.
-
[Evaluation of Naturally Occurring Compounds Regulating Brown/Beige Adipocyte Differentiation].Yakugaku Zasshi. 2019;139(6):861-866. doi: 10.1248/yakushi.18-00173-3. Yakugaku Zasshi. 2019. PMID: 31155526 Review. Japanese.
-
Adipocyte Mitochondria: Deciphering Energetic Functions across Fat Depots in Obesity and Type 2 Diabetes.Int J Mol Sci. 2024 Jun 18;25(12):6681. doi: 10.3390/ijms25126681. Int J Mol Sci. 2024. PMID: 38928386 Free PMC article. Review.
References
-
- O’Connor E.A., Evans C.V., Burda B.U., Walsh E.S., Eder M., and Lozano P. (2017). Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 317, 2427–2444. 10.1001/jama.2017.0332. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
