This is a preprint.
Mast cells promote pathology and susceptibility in tuberculosis
- PMID: 39314389
- PMCID: PMC11418949
- DOI: 10.1101/2024.09.04.611333
Mast cells promote pathology and susceptibility in tuberculosis
Abstract
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis (Mtb), infects approximately one-fourth of the world's population. While most infected individuals are asymptomatic, latent TB infection (LTBI) can progress to cause pulmonary TB (PTB). We recently reported an increased accumulation of mast cells (MCs) in lungs of macaques with PTB, compared with LTBI in macaques. MCs respond in vitro to Mtb exposure via degranulation and by inducing proinflammatory cytokines. In the current study, we show the dominant production of chymase by MCs in granulomas of humans and macaques with PTB. Using scRNA seq analysis, we show that MCs found in LTBI and healthy lungs in macaques are enriched in genes involved in tumor necrosis factor alpha, cholesterol and transforming growth factor beta signaling. In contrast, MCs clusters found in PTB express transcriptional signatures associated with interferon gamma, oxidative phosphorylation, and MYC signaling. Additionally, MC deficiency in the mouse model showed improved control of Mtb infection that coincided with reduced accumulation of lung myeloid cells and diminished inflammation at chronic stages. Thus, these collective results provide novel evidence for the pathological contribution of MCs during Mtb infection and may represent a novel target for host directive therapy for TB.
Keywords: Mast cells; Tuberculosis; inflammation; innate cell; lung.
Conflict of interest statement
Competing interests. The authors declare no competing interests.
Figures
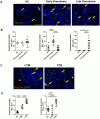
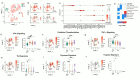

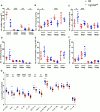
References
-
- Carlos D, Fremond C, Samarina A, Vasseur V, Maillet I, Ramos SG, Ryffel B. 2009. Histamine plays an essential regulatory role in lung inflammation and protective immunity in the acute phase of Mycobacterium tuberculosis infection. Infection and Immunity 77: 5359–5368. DOI: 10.1128/IAI.01497-08 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
