This is a preprint.
The mitochondrial dicarboxylate carrier mediates in vivo hepatic gluconeogenesis
- PMID: 39314408
- PMCID: PMC11419125
- DOI: 10.1101/2024.09.12.612761
The mitochondrial dicarboxylate carrier mediates in vivo hepatic gluconeogenesis
Abstract
Hepatic gluconeogenesis (GNG) is essential for maintaining euglycemia during prolonged fasting. However, GNG becomes pathologically elevated and drives chronic hyperglycemia in type 2 diabetes (T2D). Lactate/pyruvate is a major GNG substrate known to be imported into mitochondria for GNG. Yet, the subsequent mitochondrial carbon export mechanisms required to supply the extra-mitochondrial canonical GNG pathway have not been genetically delineated. Here, we evaluated the role of the mitochondrial dicarboxylate carrier (DiC) in mediating GNG from lactate/pyruvate. We generated liver-specific DiC knockout (DiC LivKO) mice. During lactate/pyruvate tolerance tests, DiC LivKO decreased plasma glucose excursion and 13C-lactate/-pyruvate flux into hepatic and plasma glucose. In a Western diet (WD) feeding model of T2D, acute DiC LivKO after induction of obesity decreased lactate/pyruvate-driven GNG, hyperglycemia, and hyperinsulinemia. Our results show that mitochondrial carbon export through the DiC mediates GNG and that the DiC contributes to impaired glucose homeostasis in a mouse model of T2D.
Keywords: Diabetes; Gluconeogenesis (GNG); Liver; Metabolomics; Mitochondrial dicarboxylate carrier (DiC).
Conflict of interest statement
DECLARATION OF INTERESTS EBT has consulted with BioGenerator Ventures for work unrelated to this manuscript.
Figures
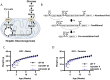
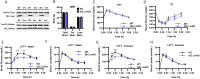
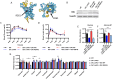
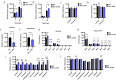

References
-
- Zoungas S., Arima H., Gerstein H.C., Holman R.R., Woodward M., Reaven P., Hayward R.A., Craven T., Coleman R.L., Chalmers J., and Collaborators on Trials of Lowering Glucose, g. (2017). Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 5, 431–437. 10.1016/S2213-8587(17)30104-3. - DOI - PubMed
-
- Zoungas S., Chalmers J., Ninomiya T., Li Q., Cooper M.E., Colagiuri S., Fulcher G., de Galan B.E., Harrap S., Hamet P., et al. (2012). Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 55, 636–643. 10.1007/s00125-011-2404-1. - DOI - PubMed
-
- Visaria J., Iyer N.N., Raval A., Kong S., Hobbs T., Bouchard J., Kern D.M., and Willey V. (2019). Incidence and Prevalence of Microvascular and Macrovascular Diseases and All-cause Mortality in Type 2 Diabetes Mellitus: A 10-year Study in a US Commercially Insured and Medicare Advantage Population. Clin Ther 41, 1522–1536 e1521. 10.1016/j.clinthera.2019.05.012. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
