This is a preprint.
Mechanisms of peptide agonist dissociation and deactivation of adhesion G-protein-coupled receptors
- PMID: 39314495
- PMCID: PMC11419055
- DOI: 10.1101/2024.09.07.611823
Mechanisms of peptide agonist dissociation and deactivation of adhesion G-protein-coupled receptors
Update in
-
Mechanisms of Peptide Agonist Dissociation and Deactivation of Adhesion G-Protein-Coupled Receptors.Biochemistry. 2025 Feb 18;64(4):871-878. doi: 10.1021/acs.biochem.4c00531. Epub 2025 Feb 4. Biochemistry. 2025. PMID: 39902762 Free PMC article.
Abstract
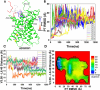
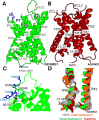
Adhesion G protein-coupled receptors (ADGRs) belong to Class B2 of GPCRs and are involved in a wide array of important physiological processes. ADGRs contain a GPCR autoproteolysis-inducing (GAIN) domain that is proximal to the receptor N-terminus and undergoes autoproteolysis during biosynthesis to generate two fragments: the N-terminal fragment (NTF) and C-terminal fragment (CTF). Dissociation of NTF reveals a tethered agonist to activate CTF of ADGRs for G protein signaling. Synthetic peptides that mimic the tethered agonist can also activate the ADGRs. However, mechanisms of peptide agonist dissociation and deactivation of ADGRs remain poorly understood. In this study, we have performed all-atom enhanced sampling simulations using a novel Protein-Protein Interaction-Gaussian accelerated Molecular Dynamics (PPI-GaMD) method on the ADGRG2-IP15 and ADGRG1-P7 complexes. The PPI-GaMD simulations captured dissociation of the IP15 and P7 peptide agonists from their target receptors. We were able to identify important low-energy conformations of ADGRG2 and ADGRG1 in the active, intermediate, and inactive states, as well as exploring different states of the peptide agonists IP15 and P7 during dissociation. Therefore, our PPI-GaMD simulations have revealed dynamic mechanisms of peptide agonist dissociation and deactivation of ADGRG1 and ADGRG2, which will facilitate rational design of peptide regulators of the two receptors and other ADGRs.
Keywords: Protein-Protein Interaction-Gaussian accelerated Molecular Dynamics; adhesion G protein-coupled receptors; deactivation; enhanced sampling; peptide dissociation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Mechanisms of Peptide Agonist Dissociation and Deactivation of Adhesion G-Protein-Coupled Receptors.Biochemistry. 2025 Feb 18;64(4):871-878. doi: 10.1021/acs.biochem.4c00531. Epub 2025 Feb 4. Biochemistry. 2025. PMID: 39902762 Free PMC article.
-
Mechanism of Tethered Agonist Binding to an Adhesion G-Protein-Coupled Receptor.bioRxiv [Preprint]. 2025 Aug 4:2025.08.04.668378. doi: 10.1101/2025.08.04.668378. bioRxiv. 2025. PMID: 40799601 Free PMC article. Preprint.
-
Mechanistic Insights into Peptide Binding and Deactivation of an Adhesion G Protein-Coupled Receptor.Molecules. 2023 Dec 27;29(1):164. doi: 10.3390/molecules29010164. Molecules. 2023. PMID: 38202747 Free PMC article.
-
Mechanisms of adhesion G protein-coupled receptor activation.J Biol Chem. 2020 Oct 9;295(41):14065-14083. doi: 10.1074/jbc.REV120.007423. Epub 2020 Aug 6. J Biol Chem. 2020. PMID: 32763969 Free PMC article. Review.
-
Sticky signaling--adhesion class G protein-coupled receptors take the stage.Sci Signal. 2013 May 21;6(276):re3. doi: 10.1126/scisignal.2003825. Sci Signal. 2013. PMID: 23695165 Review.
References
-
- Venkatakrishnan A., Deupi X., Lebon G., Tate C. G., Schertler G. F., and Babu M. M. (2013) Molecular signatures of G-protein-coupled receptors, Nature 494, 185–194. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
