Coffee intake leads to preeclampsia-like syndromes in susceptible pregnant rats
- PMID: 39314530
- PMCID: PMC11418084
- DOI: 10.1017/jns.2024.36
Coffee intake leads to preeclampsia-like syndromes in susceptible pregnant rats
Abstract
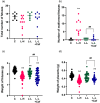
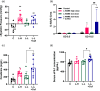
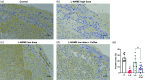
Coffee is one of the most popular beverages worldwide, and there is an increasing concern of the health risk of coffee consumption in pregnancy. Preeclampsia (PE) is a serious pregnancy disease that causes elevated blood pressure and proteinuria in pregnant women and growth restriction of fetuses due to poorly developed placental vasculature. The aim of our study is to investigate the possible effect of coffee intake during pregnancy in rats with potential underlying vasculature conditions. The endothelial nitric oxide synthase inhibitor N(gamma)-nitro-L-arginine methyl ester (L-NAME) at a high dose (125 mg/kg/d) was used to induce PE in pregnant rats, which were used as the positive control group. In addition, low-dose L-NAME (10 mg/kg/d) was used to simulate the compromised placental vasculature function in pregnant rats. Coffee was given together with low-dose L-NAME to the pregnant rats from gestational day 10.5-18.5. Our results show that the pregnant rats treated with low-dose L-NAME + coffee, but not low-dose L-NAME alone, developed PE symptoms such as prominent fetal growth restriction, hypertension, and proteinuria. Therefore, our findings suggest that coffee intake during pregnancy may cause an increased risk of PE in susceptible women.
Keywords: Coffee; Fetus; Preeclampsia; Pregnancy.
© The Author(s) 2024.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Effect and mechanism of prophylactic use of tadalafil during pregnancy on l-NAME-induced preeclampsia-like rats.Placenta. 2020 Sep 15;99:35-44. doi: 10.1016/j.placenta.2020.06.015. Epub 2020 Jul 20. Placenta. 2020. PMID: 32750643
-
Differential responses of mesenteric arterial bed to vasoactive substances in L-NAME-induced preeclampsia: Role of oxidative stress and endothelial dysfunction.Clin Exp Hypertens. 2018;40(2):126-135. doi: 10.1080/10641963.2017.1339073. Epub 2017 Jul 20. Clin Exp Hypertens. 2018. PMID: 28726518
-
Protective effect of metformin on the NG-nitro-l-arginine methyl ester (l-NAME)-induced rat models of preeclampsia.Biochem Biophys Res Commun. 2024 Dec 20;739:150996. doi: 10.1016/j.bbrc.2024.150996. Epub 2024 Nov 13. Biochem Biophys Res Commun. 2024. PMID: 39550868
-
Marked variation in responses to long-term nitric oxide inhibition during pregnancy in outbred rats from two different colonies.Am J Obstet Gynecol. 2001 Mar;184(4):686-93. doi: 10.1067/mob.2001.110448. Am J Obstet Gynecol. 2001. PMID: 11262473
-
The nitric oxide pathway in pre-eclampsia: pathophysiological implications.Hum Reprod Update. 1998 Jan-Feb;4(1):25-42. doi: 10.1093/humupd/4.1.25. Hum Reprod Update. 1998. PMID: 9622411 Review.
References
-
- Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341−354. - PubMed
-
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066−1074. - PubMed
-
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130−137. - PubMed
-
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1–544.e12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

