One-year real-world experience with mavacamten and its physiologic effects on obstructive hypertrophic cardiomyopathy
- PMID: 39314763
- PMCID: PMC11417615
- DOI: 10.3389/fcvm.2024.1429230
One-year real-world experience with mavacamten and its physiologic effects on obstructive hypertrophic cardiomyopathy
Abstract
Mavacamten is a first-in-class cardiac myosin ATPase inhibitor, approved by the United States Food and Drug Administration for the treatment of hypertrophic cardiomyopathy with obstructive physiology (oHCM). Here, we present the real-world use of mavacamten in 50 patients with oHCM at a tertiary care referral center. In both our highlighted case and in our aggregate data, we report significant improvement in wall thickness, mitral regurgitation, left ventricular outflow tract obstruction and New York Heart Association symptom class. Moreover, in our center's experience, neither arrhythmia burden, nor contractility have worsened in the vast majority of patients: we note a clinically insignificant mean decrease in left ventricular ejection fraction (LVEF), with only two patients requiring temporary mavacamten discontinuance for LVEF < 50%. Adverse events were rare, unrelated to mavacamten itself, and seen solely in patients with disease too advanced to have been represented in clinical trials. Moreover, our multidisciplinary pathway enabled us to provide a large number of patients with a novel closely-monitored therapeutic within just a few months of commercial availability. These data lead us to conclude that mavacamten, as a first-in-class cardiac myosin inhibitor, is safe and efficacious in real-world settings.
Keywords: MYK-461; cardiac myosin inhibitor; hypertrophic cardiomyopathy; hypertrophic cardiomyopathy with obstruction (oHCM); hypertrophic obstructive cardiomyopathy (HOCM); left ventricular outflow tract obstruction; mavacamten.
© 2024 Kim, Chu, Keamy-Minor, Paranjpe, Tang, O'Sullivan, Desai, Liu, Munsey, Hecker, Cuenco, Kao, Bacolor, Bonnett, Linder, Lacar, Robles, Lamendola, Smith, Knowles, Perez, Kawana, Sallam, Weldy, Wheeler, Parikh, Salisbury, Ashley and the Stanford Center for Inherited Cardiovascular Disease.
Conflict of interest statement
VP has consulting and advisory relationships with BioMarin, Lexeo Therapeutics and Viz.ai and receives funding from BioMarin, the John Taylor Babbitt Foundation. MW reports research grant and in kind support from Bristol Myers Squibb, consulting for Leal Therapeutics, outside the submitted work. EA reports advisory board fees from Apple and Foresite Labs. EA has ownership interest in SVEXA, Nuevocor, DeepCell, and Personalis, outside the submitted work. EA is a board member of AstraZeneca. CW reports consultancy fees from AiRNA Bio and Avidity Biosciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
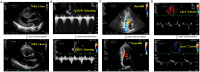
References
-
- Members WC, Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83(3):2324–405. 10.1016/j.jacc.2024.02.014 - DOI - PubMed
-
- Squibb B-M. CAMZYOS (mavacamten) REMS Program. Availability online at: https://www.camzyosrems.com (accessed November 14, 2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

