Biliary stents for active materials and surface modification: Recent advances and future perspectives
- PMID: 39314863
- PMCID: PMC11417150
- DOI: 10.1016/j.bioactmat.2024.08.031
Biliary stents for active materials and surface modification: Recent advances and future perspectives
Abstract
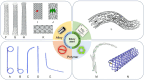
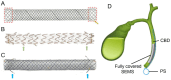
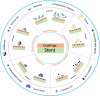
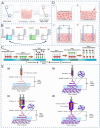
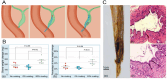
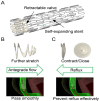
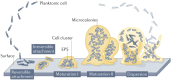
Demand for biliary stents has expanded with the increasing incidence of biliary disease. The implantation of plastic or self-expandable metal stents can be an effective treatment for biliary strictures. However, these stents are nondegradable and prone to restenosis. Surgical removal or replacement of the nondegradable stents is necessary in cases of disease resolution or restenosis. To overcome these shortcomings, improvements were made to the materials and surfaces used for the stents. First, this paper reviews the advantages and limitations of nondegradable stents. Second, emphasis is placed on biodegradable polymer and biodegradable metal stents, along with functional coatings. This also encompasses tissue engineering & 3D-printed stents were highlighted. Finally, the future perspectives of biliary stents, including pro-epithelialization coatings, multifunctional coated stents, biodegradable shape memory stents, and 4D bioprinting, were discussed.
Keywords: Biliary stent; Biodegradable; Functional coatings; Shape memory; Tissue engineering & 3D-printed stent.
© 2024 The Authors.
Conflict of interest statement
All authors declare that there are no competing interests.
Figures




Similar articles
-
Benign biliary strictures treated with biodegradable stents in patients with surgically altered anatomy using double balloon enteroscopy.Scand J Gastroenterol. 2020 Oct;55(10):1225-1233. doi: 10.1080/00365521.2020.1806351. Epub 2020 Aug 14. Scand J Gastroenterol. 2020. PMID: 32794409
-
Role of fully covered self-expandable metal stent for treatment of benign biliary strictures and bile leaks.Korean J Radiol. 2012 Jan-Feb;13 Suppl 1(Suppl 1):S67-73. doi: 10.3348/kjr.2012.13.S1.S67. Epub 2012 Apr 23. Korean J Radiol. 2012. PMID: 22563290 Free PMC article. Review.
-
Recent developments in antibacterial or antibiofilm compound coating for biliary stents.Colloids Surf B Biointerfaces. 2022 Nov;219:112837. doi: 10.1016/j.colsurfb.2022.112837. Epub 2022 Sep 10. Colloids Surf B Biointerfaces. 2022. PMID: 36137334 Review.
-
3D-printed versatile biliary stents with nanoengineered surface for anti-hyperplasia and antibiofilm formation.Bioact Mater. 2024 Mar 21;37:172-190. doi: 10.1016/j.bioactmat.2024.03.018. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 38549771 Free PMC article.
-
Biodegradable versus multiple plastic stent implantation in benign biliary strictures: A systematic review and meta-analysis.Eur J Radiol. 2020 Apr;125:108899. doi: 10.1016/j.ejrad.2020.108899. Epub 2020 Feb 13. Eur J Radiol. 2020. PMID: 32113154
Cited by
-
Recent research progresses of bioengineered biliary stents.Mater Today Bio. 2024 Oct 5;29:101290. doi: 10.1016/j.mtbio.2024.101290. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39444940 Free PMC article. Review.
-
The utilisation of biliary organoids for biomedical applications.Front Bioeng Biotechnol. 2025 Jan 7;12:1501829. doi: 10.3389/fbioe.2024.1501829. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39845376 Free PMC article. Review.
-
The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications.Materials (Basel). 2025 Apr 9;18(8):1718. doi: 10.3390/ma18081718. Materials (Basel). 2025. PMID: 40333396 Free PMC article. Review.
-
Biomedical applications of organoids derived from the digestive system.Front Cell Dev Biol. 2025 May 30;13:1599384. doi: 10.3389/fcell.2025.1599384. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519270 Free PMC article. Review.
-
Prevention of stent migration of covered self-expandable metal stents in distal malignant biliary obstruction: a review of literature.Gastroenterol Rep (Oxf). 2025 Jun 29;13:goaf058. doi: 10.1093/gastro/goaf058. eCollection 2025. Gastroenterol Rep (Oxf). 2025. PMID: 40584149 Free PMC article. Review.
References
-
- Elmunzer B.J., Maranki J.L., Gómez V., et al. A CG clinical guideline: diagnosis and management of biliary strictures. Am. J. Gastroenterol. 2023;118(3):405–426. - PubMed
-
- Choudhury S., Asthana S., Homer-Vanniasinkam S., et al. Emerging trends in biliary stents: a materials and manufacturing perspective. Biomater. Sci. 2022;10 - PubMed
-
- Chen J.-H., Wang H.-P. Endoscopic retrograde cholangiopancreatography training and education. Dig. Endosc. 2024;36(1):74–85. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

