Basiliximab induction alone vs a dual ATG-basiliximab approach in first live-donor non-sensitized kidney transplant recipients with low HLA matching
- PMID: 39314868
- PMCID: PMC11418037
- DOI: 10.1093/ckj/sfae236
Basiliximab induction alone vs a dual ATG-basiliximab approach in first live-donor non-sensitized kidney transplant recipients with low HLA matching
Erratum in
-
Correction.Clin Kidney J. 2024 Nov 20;17(11):sfae344. doi: 10.1093/ckj/sfae344. eCollection 2024 Nov. Clin Kidney J. 2024. PMID: 39569315 Free PMC article.
Abstract
Background: Individualizing induction therapy based on immunological risk is crucial for optimizing outcomes in kidney transplantation.
Methods: A retrospective analysis included 157 first live-donor non-sensitized kidney transplant recipients (KTRs). Within this cohort, 96 individuals exhibited low human leukocyte antigen (HLA) matching (5-6 HLA mismatches). The low HLA match subgroup was categorized into 52 KTRs receiving basiliximab alone and 44 recipients treated with a combined single ATG dose of 1.5 mg/kg and basiliximab. The primary endpoint was early acute cellular rejection (ACR) within 6 months post-transplant while secondary outcomes encompassed infection rates, renal allograft function, length of stay (LOS) and readmissions post-transplant.
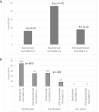
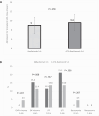
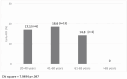
Results: The incidence of early ACR was decreased for low HLA match KTRs, who received ATG-basiliximab, when compared with low HLA-matched KTRs who received basiliximab alone (9.1% vs 23.9%, P = .067). Age was a predictor for rejection, and subgroup analysis showed consistent rejection reduction across age groups. No significant differences were observed in admission for transplant LOS or in peri-operative complications, nor in infections rate including BK and cytomegalovirus viremia, allograft function and number of readmissions post-transplant up to 6 months post-transplant.
Conclusion: In non-sensitized first live-donor KTRs with low HLA matching, a dual ATG-basiliximab induction approach significantly reduced early ACR without compromising safety.
Keywords: HLA match; anti-thymocyte globulin (ATG); basiliximab; induction treatment; kidney transplantation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A retrospective comparison of the efficacy and safety in kidney transplant recipients with basiliximab and anti-thymocyte globulin.Chin Med J (Engl). 2012 Mar;125(6):1135-40. Chin Med J (Engl). 2012. PMID: 22613543
-
Assessment of infectious complications in elderly kidney transplant recipients receiving induction with anti-thymocyte globulin vs basiliximab.Transpl Infect Dis. 2020 Jun;22(3):e13257. doi: 10.1111/tid.13257. Epub 2020 Mar 6. Transpl Infect Dis. 2020. PMID: 32031729
-
Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial.Lancet. 2016 Dec 17;388(10063):3006-3016. doi: 10.1016/S0140-6736(16)32187-0. Epub 2016 Nov 19. Lancet. 2016. PMID: 27871759 Clinical Trial.
-
Basiliximab: a review of its use as induction therapy in renal transplantation.Drugs. 2003;63(24):2803-35. doi: 10.2165/00003495-200363240-00009. Drugs. 2003. PMID: 14664658 Review.
-
Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection.Drugs. 2009 Jul 30;69(11):1483-512. doi: 10.2165/00003495-200969110-00007. Drugs. 2009. PMID: 19634926 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

