Low-Volume Ex Situ Lung Perfusion System for Single Lung Application in a Small Animal Model Enables Optimal Compliance With " Reduction" in 3R Principles of Animal Research
- PMID: 39314923
- PMCID: PMC11418019
- DOI: 10.3389/ti.2024.13189
Low-Volume Ex Situ Lung Perfusion System for Single Lung Application in a Small Animal Model Enables Optimal Compliance With " Reduction" in 3R Principles of Animal Research
Abstract
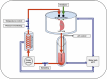
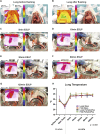
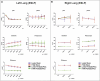
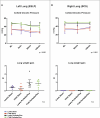
Ex situ lung perfusion (ESLP) is used for organ reconditioning, repair, and re-evaluation prior to transplantation. Since valid preclinical animal models are required for translationally relevant studies, we developed a 17 mL low-volume ESLP for double- and single-lung application that enables cost-effective optimal compliance "reduction" of the 3R principles of animal research. In single-lung mode, ten Fischer344 and Lewis rat lungs were subjected to ESLP and static cold storage using STEEN or PerfadexPlus. Key perfusion parameters, thermal lung imaging, blood gas analysis (BGA), colloid oncotic pressure (COP), lung weight gain, histological work-up, and cytokine analysis were performed. Significant differences between perfusion solutions but not between the rat strains were detected. Most relevant perfusion parameters confirmed valid ESLP with homogeneous lung perfusion, evidenced by uniform lung surface temperature. BGA showed temperature-dependent metabolic activities with differences depending on perfusion solution composition. COP is not decisive for pulmonary oedema and associated weight gain, but possibly rather observed chemokine profile and dextran sensitivity of rats. Histological examination confirmed intact lung architecture without infarcts or hemorrhages due to optimal organ procurement and single-lung application protocol using our in-house-designed ESLP system.
Keywords: ex situ lung perfusion/ESLP; ex vivo lung perfusion/EVLP; rat model; single lung; small animal model.
Copyright © 2024 Katsirntaki, Hagner, Werlein, Braubach, Jonigk, Adam, Hidaji, Kühn, Falk, Ruhparwar and Wiegmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation.Cochrane Database Syst Rev. 2024 Jul 9;7(7):CD011671. doi: 10.1002/14651858.CD011671.pub3. Cochrane Database Syst Rev. 2024. PMID: 38979743 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Preservation of bronchial artery circulation on ex-vivo lung perfusion.Sci Rep. 2025 Jul 2;15(1):23354. doi: 10.1038/s41598-025-06174-8. Sci Rep. 2025. PMID: 40603432 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Ex vivo lung perfusion-to-lung transplant rat survival model with reproducible development of acute lung injury and graft rejection.JHLT Open. 2025 May 26;9:100299. doi: 10.1016/j.jhlto.2025.100299. eCollection 2025 Aug. JHLT Open. 2025. PMID: 40530004 Free PMC article.
References
-
- Warnecke G, Moradiellos J, Tudorache I, Kühn C, Avsar M, Wiegmann B, et al. Normothermic Perfusion of Donor Lungs for Preservation and Assessment With the Organ Care System Lung Before Bilateral Transplantation: A Pilot Study of 12 Patients. Lancet (2012) 380(9856):1851–8. 10.1016/S0140-6736(12)61344-0 - DOI - PubMed
-
- Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F, et al. Normothermic Ex-Vivo Preservation With Portable Organ Care System Lung Device for Bilateral Lung Transplantation (INSPIRE): A Randomoised, Open-Label, Non-Inferiority, Phase 3 Study. Lancet Respir Med (2018) 6(5):357–67. 10.1016/S2213-2600(18)30136-X - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

