This is a preprint.
Reconciling heterogeneous dengue virus infection risk estimates from different study designs
- PMID: 39314937
- PMCID: PMC11419196
- DOI: 10.1101/2024.09.09.24313375
Reconciling heterogeneous dengue virus infection risk estimates from different study designs
Update in
-
Reconciling heterogeneous dengue virus infection risk estimates from different study designs.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2411768121. doi: 10.1073/pnas.2411768121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739790 Free PMC article.
Abstract
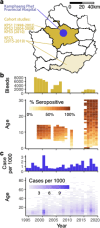
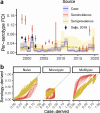
Uncovering rates at which susceptible individuals become infected with a pathogen, i.e. the force of infection (FOI), is essential for assessing transmission risk and reconstructing distribution of immunity in a population. For dengue, reconstructing exposure and susceptibility statuses from the measured FOI is of particular significance as prior exposure is a strong risk factor for severe disease. FOI can be measured via many study designs. Longitudinal serology are considered gold standard measurements, as they directly track the transition of seronegative individuals to seropositive due to incident infections (seroincidence). Cross-sectional serology can provide estimates of FOI by contrasting seroprevalence across ages. Age of reported cases can also be used to infer FOI. Agreement of these measurements, however, have not been assessed. Using 26 years of data from cohort studies and hospital-attended cases from Kamphaeng Phet province, Thailand, we found FOI estimates from the three sources to be highly inconsistent. Annual FOI estimates from seroincidence was 2.46 to 4.33-times higher than case-derived FOI. Correlation between seroprevalence-derived and case-derived FOI was moderate (correlation coefficient=0.46) and no systematic bias. Through extensive simulations and theoretical analysis, we show that incongruences between methods can result from failing to account for dengue antibody kinetics, assay noise, and heterogeneity in FOI across ages. Extending standard inference models to include these processes reconciled the FOI and susceptibility estimates. Our results highlight the importance of comparing inferences across multiple data types to uncover additional insights not attainable through a single data type/analysis.
Keywords: catalytic model; dengue force of infection; seroincidence; seroprevalence.
Figures


Similar articles
-
Reconciling heterogeneous dengue virus infection risk estimates from different study designs.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2411768121. doi: 10.1073/pnas.2411768121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739790 Free PMC article.
-
Estimating the annual dengue force of infection from the age of reporting primary infections across urban centres in endemic countries.BMC Med. 2021 Sep 30;19(1):217. doi: 10.1186/s12916-021-02101-6. BMC Med. 2021. PMID: 34587957 Free PMC article.
-
Dynamics and determinants of the force of infection of dengue virus from 1994 to 2015 in Managua, Nicaragua.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10762-10767. doi: 10.1073/pnas.1809253115. Epub 2018 Sep 28. Proc Natl Acad Sci U S A. 2018. PMID: 30266790 Free PMC article.
-
A scoping literature review of global dengue age-stratified seroprevalence data: estimating dengue force of infection in endemic countries.EBioMedicine. 2024 Jun;104:105134. doi: 10.1016/j.ebiom.2024.105134. Epub 2024 May 7. EBioMedicine. 2024. PMID: 38718682 Free PMC article.
-
Age-Dependent Risk of Respiratory Syncytial Virus Infection: A Systematic Review and Hazard Modeling From Serological Data.J Infect Dis. 2023 Nov 11;228(10):1400-1409. doi: 10.1093/infdis/jiad147. J Infect Dis. 2023. PMID: 37161934 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
