This is a preprint.
Fc-enhanced anti-CTLA-4 depletes tumor-infiltrating regulatory T cells to augment immune effects of androgen ablation in high-risk prostate cancer
- PMID: 39314954
- PMCID: PMC11419205
- DOI: 10.1101/2024.09.09.24313308
Fc-enhanced anti-CTLA-4 depletes tumor-infiltrating regulatory T cells to augment immune effects of androgen ablation in high-risk prostate cancer
Abstract
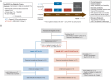
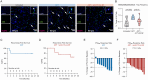
Despite high rates of post-surgical recurrence in men with high-risk localized prostate cancer (PCa), there is currently no role for neoadjuvant therapy. Tumor infiltrating regulatory T cells (TI-Tregs) limit the antitumor effects of presurgical androgen deprivation therapy (ADT). Therefore, we designed a neoadjuvant clinical trial to test whether Treg depletion via a non-fucosylated anti-CTLA-4 antibody (BMS-986218) is feasible and augments response to ADT. In this single-center, two-arm, open-label study, 24 men with high-risk localized PCa were randomized to ADT with or without BMS-986218 prior to radical prostatectomy. Treatment was well tolerated and feasible. Mechanistic studies indicated BMS-986218 depleted TI-Tregs by engaging CD16a/FCGR3A on tumor macrophages, modulated dendritic cells (DCs), and augmented T cell priming. Depth of Treg depletion and increased DC frequencies were quantitatively associated with improved clinical outcome. Overall, this study supports the feasibility and biological activity of neoadjuvant immunotherapy with ADT + Fc-enhanced anti-CTLA-4 in high-risk localized PCa.
Keywords: CTLA-4; CyTOF; Ipilimumab; Neoadjuvant; Prostate Cancer; Regulatory T Cell; single cell RNA sequencing.
Conflict of interest statement
Conflicts of Interest: C.G.D is a co-inventor on patents licensed from Johns Hopkins University to BMS and Janssen. He is currently a paid employee of JnJ Innovative Medicine. A.C. is founder, equity holder, consultant, and director of DarwinHealth Inc., which has licensed IP related to these algorithms from Columbia University. Columbia University is an equity holder in DarwinHealth Inc.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
