This is a preprint.
Clinical Trials for Wolfram Syndrome Neurodegeneration: Novel Design, Endpoints, and Analysis Models
- PMID: 39314971
- PMCID: PMC11419225
- DOI: 10.1101/2024.09.10.24313426
Clinical Trials for Wolfram Syndrome Neurodegeneration: Novel Design, Endpoints, and Analysis Models
Update in
-
Clinical trials for Wolfram syndrome neurodegeneration: Novel design, endpoints, and analysis models.PLoS One. 2025 May 9;20(5):e0321598. doi: 10.1371/journal.pone.0321598. eCollection 2025. PLoS One. 2025. PMID: 40344084 Free PMC article.
Abstract
Objective: Wolfram syndrome, an ultra-rare condition, currently lacks effective treatment options. The rarity of this disease presents significant challenges in conducting clinical trials, particularly in achieving sufficient statistical power (e.g., 80%). The objective of this study is to propose a novel clinical trial design based on real-world data to reduce the sample size required for conducting clinical trials for Wolfram syndrome.
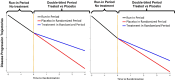
Methods: We propose a novel clinical trial design with three key features aimed at reducing sample size and improve efficiency: (i) Pooling historical/external controls from a longitudinal observational study conducted by the Washington University Wolfram Research Clinic. (ii) Utilizing run-in data to estimate model parameters. (iii) Simultaneously tracking treatment effects in two endpoints using a multivariate proportional linear mixed effects model.
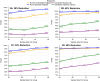
Results: Comprehensive simulations were conducted based on real-world data obtained through the Wolfram syndrome longitudinal observational study. Our simulations demonstrate that this proposed design can substantially reduce sample size requirements. Specifically, with a bivariate endpoint and the inclusion of run-in data, a sample size of approximately 30 per group can achieve over 80% power, assuming the placebo progression rate remains consistent during both the run-in and randomized periods. In cases where the placebo progression rate varies, the sample size increases to approximately 50 per group.
Conclusions: For rare diseases like Wolfram syndrome, leveraging existing resources such as historical/external controls and run-in data, along with evaluating comprehensive treatment effects using bivariate/multivariate endpoints, can significantly expedite the development of new drugs.
Keywords: Wolfram syndrome; historical/external controls; multivariate endpoints; multivariate proportional model; run-in data.
Conflict of interest statement
Competing interests: None
Figures

Similar articles
-
Clinical trials for Wolfram syndrome neurodegeneration: Novel design, endpoints, and analysis models.PLoS One. 2025 May 9;20(5):e0321598. doi: 10.1371/journal.pone.0321598. eCollection 2025. PLoS One. 2025. PMID: 40344084 Free PMC article.
-
Longitudinal progression of diabetes mellitus in Wolfram syndrome: The Washington University Wolfram Research Clinic experience.Pediatr Diabetes. 2022 Mar;23(2):212-218. doi: 10.1111/pedi.13291. Epub 2021 Dec 19. Pediatr Diabetes. 2022. PMID: 34792267 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Review of Acute Treatment of Migraine Trial Results With the New FDA Endpoints: Design Implications for Future Trials.Headache. 2019 May;59(5):819-824. doi: 10.1111/head.13511. Epub 2019 Apr 6. Headache. 2019. PMID: 30953576 Free PMC article. Review.
-
Current Landscape of Treatments for Wolfram Syndrome.Trends Pharmacol Sci. 2019 Oct;40(10):711-714. doi: 10.1016/j.tips.2019.07.011. Epub 2019 Aug 13. Trends Pharmacol Sci. 2019. PMID: 31420094 Free PMC article. Review.
Cited by
-
Determination of the role of miR-451a on Plasmodium falciparum red blood cell stages, oxidative stress, and proteomic profiling.Mol Biol Rep. 2024 Oct 7;51(1):1041. doi: 10.1007/s11033-024-09938-z. Mol Biol Rep. 2024. PMID: 39373748
References
-
- DJ W. Diabetes mellitus and simple optic atrophy among siblings: Report of 4 cases. in Mayo Clin Proc. 1938.
-
- Barrett T.G., Bundey S.E., and Macleod A.F., Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. The Lancet, 1995. 346(8988): p. 1458–1463. - PubMed
-
- Inoue H., et al., A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nature genetics, 1998. 20(2): p. 143–148. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
