MA'AT analysis of the O-glycosidic linkages of oligosaccharides using nonconventional NMR J-couplings: MA'AT and MD models of phi
- PMID: 39315028
- PMCID: PMC11418834
- DOI: 10.1039/d4ra06062h
MA'AT analysis of the O-glycosidic linkages of oligosaccharides using nonconventional NMR J-couplings: MA'AT and MD models of phi
Abstract
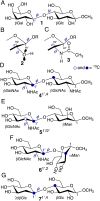
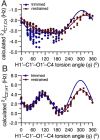
MA'AT analysis (Meredith et al., J. Chem. Inf. Model. 2022, 62, 3135-3141) is a new NMR-based method to treat ensembles of redundant NMR spin-coupling constants (J-couplings) to obtain experiment-based probability distributions of molecular torsion angles in solution. Work reported to date on modeling the conformations of O-glycosidic linkages of oligosaccharides using three conventional J-coupling constraints (2 J COC, 3 J COCH, 3 J COCC) has shown that the method gives mean torsion angles and circular standard deviations (CSDs) for psi in very good agreement with those obtained by MD simulation. On the other hand, CSDs for phi determined by MA'AT analysis have consistently been much larger than those determined by MD, calling into question either the reliability of MA'AT analysis or MD to accurately predict this behavior. Prior work has shown that this discrepancy does not stem from the limitations of DFT-based J-coupling equation parameterization where secondary conformational dependencies can introduce uncertainties. The present work re-visits this problem by incorporating a new nonconventional J-coupling constraint into MA'AT analyses of phi, namely, a geminal (two-bond) 2 J CCH J-value that exhibits a strong primary dependence on phi. The latter property pertains explicitly to linkages contributed by GlcNAc pyranosyl rings and pyranosyl rings devoid of substituents at C2 (i.e., deoxy residues) where known secondary contributions to 2 J CCH magnitude caused by C-O bond rotation involving the coupled carbon are negligible or absent. The results show that when 2 J CCH values are added to the analysis, phi CSDs reduce considerably, bringing them into better alignment with those obtained by MD simulation. The cause of the discrepancy when only three conventional J-couplings are used to treat phi appears to be associated with the two-bond 2 J COC, which has properties that make it less effective than the non-conventional 2 J CCH as a discriminator of different conformational models of phi.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Zhang W. Meredith R. Pan Q. Wang X. Woods R. J. Carmichael I. Serianni A. S. Use of circular statistics to model αMan-(1→2)-αMan and αMan-(1→3)-α/βMan O-glycosidic linkage conformation in 13C-labeled disaccharides and high-mannose oligosaccharides. Biochemistry. 2019;58:546–560. - PubMed
-
- Meredith R. J. Carmichael I. Woods R. J. Serianni A. S. MA’AT analysis: Probability distributions of molecular torsion angles in solution from NMR spectroscopy. Acc. Chem. Res. 2023;56:2313–2328. - PubMed
-
- Tvaroska I. Hricovini H. Petrakova E. An attempt to derive a new Karplus-type equation of vicinal proton-carbon coupling constants for C–O–C–H segments of bonded atoms. Carbohydr. Res. 1989;189:359–362.
-
- Mulloy B. Frenkiel T. A. Davies D. B. Long-range carbon-proton coupling constants: Application to conformational studies of oligosaccharides. Carbohydr. Res. 1988;184:39–46. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

