Causal associations between gut microbiota, circulating inflammatory proteins, and epilepsy: a multivariable Mendelian randomization study
- PMID: 39315097
- PMCID: PMC11416947
- DOI: 10.3389/fimmu.2024.1438645
Causal associations between gut microbiota, circulating inflammatory proteins, and epilepsy: a multivariable Mendelian randomization study
Abstract
Background: Previous studies have suggested that gut microbiota (GM) may be involved in the pathogenesis of epilepsy through the microbiota-gut-brain axis (MGBA). However, the causal relationship between GM and different epilepsy subtypes and whether circulating inflammatory proteins act as mediators to participate in epileptogenesis through the MGBA remain unclear. Therefore, it is necessary to identify specific GM associated with epilepsy and its subtypes and explore their underlying inflammatory mechanisms for risk prediction, personalized treatment, and prognostic monitoring of epilepsy.
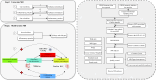
Methods: We hypothesized the existence of a pathway GM-inflammatory proteins-epilepsy. We found genetic variants strongly associated with GM, circulating inflammatory proteins, epilepsy and its subtypes, including generalized and partial seizures, from large-scale genome-wide association studies (GWAS) summary data and used Multivariate Mendelian Randomization to explore the causal relationship between the three and whether circulating inflammatory proteins play a mediating role in the pathway from GM to epilepsy, with inverse variance weighted (IVW) method as the primary statistical method, supplemented by four methods: MR-Egger, weighted median estimator (WME), Weighted mode and Simple mode.
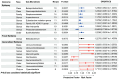
Results: 16 positive and three negative causal associations were found between the genetic liability of GM and epilepsy and its subtypes. There were nine positive and nine negative causal associations between inflammatory proteins and epilepsy and its subtypes. Furthermore, we found that C-X-C motif chemokine 11 (CXCL11) levels mediated the causal association between Genus Family XIII AD3011 group and epilepsy.
Conclusion: Our study highlights the possible causal role of specific GM and specific inflammatory proteins in the development of epilepsy and suggests that circulating inflammatory proteins may mediate epileptogenesis through the MGBA.
Keywords: Mendelian randomization; epilepsy; gut microbiota; inflammatory proteins; microbiota-gut-brain-axis.
Copyright © 2024 Yang, Liu, Gao, Liu, Zhang, Liu, Ma, Zhang, Shi, Duan, Ma, Zhang, Cheng, Qu, Chen and Zhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Gut microbiota, circulating inflammatory proteins and sepsis: a bi-directional Mendelian randomization study.Front Cell Infect Microbiol. 2024 Aug 8;14:1398756. doi: 10.3389/fcimb.2024.1398756. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39176264 Free PMC article.
-
The mediating effect of circulating inflammatory proteins on the relationship between gut microbiota and FD: a bidirectional Mendelian randomization study.Sci Rep. 2024 Oct 11;14(1):23785. doi: 10.1038/s41598-024-74717-6. Sci Rep. 2024. PMID: 39390038 Free PMC article.
-
Genetic evidence supporting the causal role of gut microbiota in chronic kidney disease and chronic systemic inflammation in CKD: a bilateral two-sample Mendelian randomization study.Front Immunol. 2023 Nov 2;14:1287698. doi: 10.3389/fimmu.2023.1287698. eCollection 2023. Front Immunol. 2023. PMID: 38022507 Free PMC article.
-
Genetic liability of gut microbiota for idiopathic pulmonary fibrosis and lung function: a two-sample Mendelian randomization study.Front Cell Infect Microbiol. 2024 May 22;14:1348685. doi: 10.3389/fcimb.2024.1348685. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38841114 Free PMC article.
-
Exploring the potential causal relationship between gut microbiota and heart failure: A two-sample mendelian randomization study combined with the geo database.Curr Probl Cardiol. 2024 Feb;49(2):102235. doi: 10.1016/j.cpcardiol.2023.102235. Epub 2023 Nov 30. Curr Probl Cardiol. 2024. PMID: 38040216 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

