The potential of autologous regulatory T cell (Treg) therapy to prevent Cardiac Allograft Vasculopathy (CAV) in paediatric heart transplant recipients
- PMID: 39315099
- PMCID: PMC11416935
- DOI: 10.3389/fimmu.2024.1444924
The potential of autologous regulatory T cell (Treg) therapy to prevent Cardiac Allograft Vasculopathy (CAV) in paediatric heart transplant recipients
Abstract
Paediatric heart transplant is an established treatment for end stage heart failure in children, however patients have to commit to lifelong medical surveillance and adhere to daily immunosuppressants to minimise the risk of rejection. Compliance with immunosuppressants can be burdensome with their toxic side effects and need for frequent blood monitoring especially in children. Though the incidence of early rejection episodes has significantly improved overtime, the long-term allograft health and survival is determined by Cardiac Allograft Vasculopathy (CAV) which affects a vast number of post-transplant patients. Once CAV has set in, there is no medical or surgical treatment to reverse it and graft survival is significantly compromised across all age groups. Current treatment strategies include novel immunosuppressant agents and drugs to lower blood lipid levels to address the underlying immunological pathophysiology and to manage traditional cardiac risk factors. Translational researchers are seeking novel immunological approaches that can lead to permanent acceptance of the allograft such as using regulatory T cell (Tregs) immunotherapy. Clinical trials in the setting of graft versus host disease, autoimmunity and kidney and liver transplantation using Tregs have shown the feasibility and safety of this strategy. This review will summarise current knowledge of the latest clinical therapies for CAV and pre-clinical evidence in support of Treg therapy for CAV. We will also discuss the different Treg sources and the considerations of translating this into a feasible immunotherapy in clinical practice in the paediatric population.
Keywords: cardiac allograft vasculopathy (CAV); cell therapy; paediatric; regulatory T cells; thymic; transplantation.
Copyright © 2024 Aiyengar, Romano, Burch, Lombardi and Fanelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
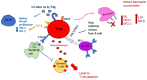
Figures

References
-
- Rossano JW, Singh TP, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Jr., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report - 2019; Focus theme: Donor and recipient size match. J Heart Lung Transpl. (2019) 38:1028–41. doi: 10.1016/j.healun.2019.08.002 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

