Systemic immunostimulation induces glucocorticoid-mediated thymic involution succeeded by rebound hyperplasia which is impaired in aged recipients
- PMID: 39315105
- PMCID: PMC11416920
- DOI: 10.3389/fimmu.2024.1429912
Systemic immunostimulation induces glucocorticoid-mediated thymic involution succeeded by rebound hyperplasia which is impaired in aged recipients
Abstract
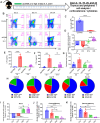
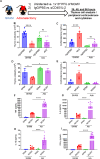
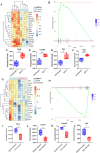
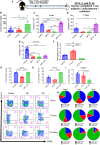
The thymus is the central organ involved with T-cell development and the production of naïve T cells. During normal aging, the thymus undergoes marked involution, reducing naïve T-cell output and resulting in a predominance of long-lived memory T cells in the periphery. Outside of aging, systemic stress responses that induce corticosteroids (CS), or other insults such as radiation exposure, induce thymocyte apoptosis, resulting in a transient acute thymic involution with subsequent recovery occurring after cessation of the stimulus. Despite the increasing utilization of immunostimulatory regimens in cancer, effects on the thymus and naïve T cell output have not been well characterized. Using both mouse and human systems, the thymic effects of systemic immunostimulatory regimens, such as high dose IL-2 (HD IL-2) with or without agonistic anti-CD40 mAbs and acute primary viral infection, were investigated. These regimens produced a marked acute thymic involution in mice, which correlated with elevated serum glucocorticoid levels and a diminishment of naïve T cells in the periphery. This effect was transient and followed with a rapid thymic "rebound" effect, in which an even greater quantity of thymocytes was observed compared to controls. Similar results were observed in humans, as patients receiving HD IL-2 treatment for cancer demonstrated significantly increased cortisol levels, accompanied by decreased peripheral blood naïve T cells and reduced T-cell receptor excision circles (TRECs), a marker indicative of recent thymic emigrants. Mice adrenalectomized prior to receiving immunotherapy or viral infection demonstrated protection from this glucocorticoid-mediated thymic involution, despite experiencing a substantially higher inflammatory cytokine response and increased immunopathology. Investigation into the effects of immunostimulation on middle aged (7-12 months) and advance aged (22-24 months) mice, which had already undergone significant thymic involution and had a diminished naïve T cell population in the periphery at baseline, revealed that even further involution was incurred. Thymic rebound hyperplasia, however, only occurred in young and middle-aged recipients, while advance aged not only lacked this rebound hyperplasia, but were entirely absent of any indication of thymic restoration. This coincided with prolonged deficits in naïve T cell numbers in advanced aged recipients, further skewing the already memory dominant T cell pool. These results demonstrate that, in both mice and humans, systemic immunostimulatory cancer therapies, as well as immune challenges like subacute viral infections, have the potential to induce profound, but transient, glucocorticoid-mediated thymic involution and substantially reduced thymic output, resulting in the reduction of peripheral naive T cells. This can then be followed by a marked rebound effect with naïve T cell restoration, events that were shown not to occur in advanced-aged mice.
Keywords: age; glucocoricoids; immune therapy; stress; thymic involution; viral infection.
Copyright © 2024 Collins, Khuat, Sckisel, Vick, Minnar, Dunai, Le, Curti, Crittenden, Merleev, Sheng, Chao, Maverakis, Rosario, Monjazeb, Blazar, Longo, Canter and Murphy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Glucocorticoids impair T lymphopoiesis after myocardial infarction.Am J Physiol Heart Circ Physiol. 2024 Aug 1;327(2):H533-H544. doi: 10.1152/ajpheart.00195.2024. Epub 2024 Jul 12. Am J Physiol Heart Circ Physiol. 2024. PMID: 38995212 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

