Pseudomonas aeruginosa exotoxin A as a novel allergen induced Non-TH2 inflammation in a murine model of steroid-insensitive asthma
- PMID: 39315215
- PMCID: PMC11417555
- DOI: 10.1016/j.heliyon.2024.e37512
Pseudomonas aeruginosa exotoxin A as a novel allergen induced Non-TH2 inflammation in a murine model of steroid-insensitive asthma
Abstract
Background: Despite the immediate in vivo occurrence of anaphylactic and allergic reactions following treatment with Pseudomonas aeruginosa exotoxin A (PEA)-based immunotoxins, the immunological role of PEA in asthma pathogenesis remains unclear.
Objective: This study investigated the allergenic potential of PEA and the specific type of asthma induced.
Methods: Recombinant PEA (rPEA) lacking domain Ia (to eliminate non-specific cytotoxicity) was expressed, purified, and employed to detect serum PEA-specific IgE levels in asthmatic patients. Competitive ELISA assays were used to assess rPEA's IgE binding capacity and allergenicity. Additionally, rPEA-challenged C57BL/6 mice were subjected to inflammatory endotyping and therapeutic assays to characterize the allergic nature of PEA.
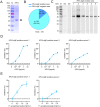
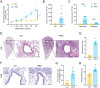
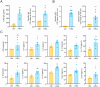
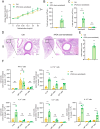
Results: PEA-specific IgE was identified in 17 (14.2 %) of 120 asthma patients. The rPEA-sensitized and challenged mice had increased PEA-specific immunoglobulins (such as IgE, IgG1 and IgG2a) and developed asthma-like phenotypes with airway hyperresponsiveness, severe airway inflammation, and airway remodeling. Lungs from these mice displayed significant increases in neutrophils and IL-17A+ cells. Innate lymphoid cells (ILCs) produced type 2 cytokines (IL-4, IL-5, and IL-13), whereas Th cells did not. Nonetheless, airway inflammation, rather than hyperresponsiveness, was elicited in non-sensitized mice upon challenge with rPEA. Importantly, rPEA-induced asthmatic mice were unresponsive to dexamethasone treatment.
Conclusion: PEA is a novel allergen that sensitizes asthmatic patients. Furthermore, mice developed steroid-resistant asthma, characterized by an atypical cytokine profile associated with non-TH2 inflammation, only after being sensitized and challenged with rPEA. These findings suggest a potentially significant role for PEA in asthma development, warranting consideration in clinical diagnosis and treatment strategies.
Keywords: Allergen; Asthma; IL-17A; Pseudomonas aeruginosa exotoxin A; Steroid-insensitive.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Transcription factors GATA-3 and RORγt are important for determining the phenotype of allergic airway inflammation in a murine model of asthma.J Immunol. 2013 Feb 1;190(3):1056-65. doi: 10.4049/jimmunol.1202386. Epub 2013 Jan 4. J Immunol. 2013. PMID: 23293351
-
Development of a novel severe triple allergen asthma model in mice which is resistant to dexamethasone and partially resistant to TLR7 and TLR9 agonist treatment.PLoS One. 2014 Mar 11;9(3):e91223. doi: 10.1371/journal.pone.0091223. eCollection 2014. PLoS One. 2014. PMID: 24618687 Free PMC article.
-
Semaphorin 7A plays a critical role in IgE-mediated airway inflammation in mice.Eur J Pharmacol. 2015 Oct 5;764:149-156. doi: 10.1016/j.ejphar.2015.07.004. Epub 2015 Jul 2. Eur J Pharmacol. 2015. PMID: 26144372
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Innate lymphoid cells in asthma: pathophysiological insights from murine models to human asthma phenotypes.Curr Opin Allergy Clin Immunol. 2019 Feb;19(1):53-60. doi: 10.1097/ACI.0000000000000497. Curr Opin Allergy Clin Immunol. 2019. PMID: 30516548 Review.
References
LinkOut - more resources
Full Text Sources

