AGBL2 promotes renal cell carcinoma cells proliferation and migration via α-tubulin detyrosination
- PMID: 39315218
- PMCID: PMC11417249
- DOI: 10.1016/j.heliyon.2024.e37086
AGBL2 promotes renal cell carcinoma cells proliferation and migration via α-tubulin detyrosination
Abstract
Background: AGBL2's role in tumorigenesis and cancer progression has been reported in several cancer studies, and it is closely associated with α-tubulin detyrosination. The roles of AGBL2 and α-tubulin detyrosination in renal cell carcinoma (RCC) pathogenesis remain unclear and require further investigation.
Methods: In this study, we conducted an analysis of AGBL2 expression differences between renal clear cell carcinoma tissues and normal tissues using data from The Cancer Genome Atlas (TCGA). We performed a comprehensive prognostic analysis of AGBL2 in Kidney Renal Clear Cell Carcinoma (KIRC) using univariate and multivariate Cox regression. Based on the results of the Cox analysis, we constructed a prognostic model to assess its predictive capabilities. Receiver Operating Characteristic (ROC) analysis confirmed the diagnostic value of AGBL2 in renal cancer. We conducted further validation by analyzing cancer tissue samples and renal cancer cell lines, which confirmed the role of AGBL2 in promoting RCC cell proliferation and migration through in vitro experiments. Additionally, we verified the impact of AGBL2's detyrosination on α-tubulin using the tubulin carboxypeptidase (TCP) inhibitor parthenolide. Finally, we performed sequencing analysis on AGBL2 knockdown 786-O cells to investigate the correlation between AGBL2, immune infiltration, and AKT phosphorylation. Moreover, we experimentally demonstrated the enhancing effect of AGBL2 on AKT phosphorylation.
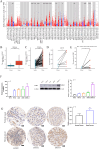
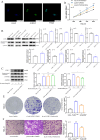
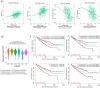
Results: TCGA analysis revealed a significant increase in AGBL2 expression in RCC patients, which was correlated with poorer overall survival (OS), disease-specific survival (DSS), and progression-free intervals (PFI). According to the analysis results, we constructed column-line plots to predict the 1-, 3-, and 5-year survival outcomes in RCC patients. Additionally, the calibration plots assessing the model's performance exhibited favorable agreement with the predicted outcomes. And the ROC curves showed that AGBL2 showed good diagnostic performance in KIRC (AUC = 0.836)). Cell phenotyping assays revealed that AGBL2 knockdown in RCC cells significantly inhibited cell proliferation and migration. Conversely, overexpression of AGBL2 resulted in increased cell proliferation and migration in RCC cells. We observed that AGBL2 is predominantly located in the nucleus and can elevate the detyrosination level of α-tubulin in RCC cells. Moreover, the enhancement of RCC cell proliferation and migration by AGBL2 was partially inhibited after treatment with the TCP inhibitor parthenolide. Analysis of the sequencing data revealed that AGBL2 is associated with a diverse array of biological processes, encompassing signal transduction and immune infiltration. Interestingly, AGBL2 expression exhibited a negative correlation with the majority of immune cell infiltrations. Additionally, AGBL2 was found to enhance the phosphorylation of AKT in RCC cells.
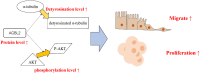
Conclusion: Our study suggests that AGBL2 fosters RCC cell proliferation and migration by enhancing α-tubulin detyrosination. Moreover, elevated AGBL2 expression increases phosphorylation of AKT in RCC cells.
Keywords: AGBL2; Detyrosination; Microtubules; Renal cell carcinoma.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Calbindin S100A16 Promotes Renal Cell Carcinoma Progression and Angiogenesis via the VEGF/VEGFR2 Signaling Pathway.Contrast Media Mol Imaging. 2022 Sep 15;2022:5602011. doi: 10.1155/2022/5602011. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36176934 Free PMC article.
-
AGBL2 promotes cancer cell growth through IRGM-regulated autophagy and enhanced Aurora A activity in hepatocellular carcinoma.Cancer Lett. 2018 Feb 1;414:71-80. doi: 10.1016/j.canlet.2017.11.003. Epub 2017 Nov 8. Cancer Lett. 2018. PMID: 29126912
-
Glutathione peroxidase family and survival prognosis in patients with renal cell carcinoma.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 May 28;47(5):562-572. doi: 10.11817/j.issn.1672-7347.2022.210418. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35753726 Free PMC article. Chinese, English.
-
High expression of AGBL2 is a novel prognostic factor of adverse outcome in patients with ovarian carcinoma.Oncol Lett. 2019 Nov;18(5):4900-4906. doi: 10.3892/ol.2019.10829. Epub 2019 Sep 9. Oncol Lett. 2019. PMID: 31612000 Free PMC article.
-
Characterization of the basement membrane in kidney renal clear cell carcinoma to guide clinical therapy.Front Oncol. 2022 Nov 10;12:1024956. doi: 10.3389/fonc.2022.1024956. eCollection 2022. Front Oncol. 2022. PMID: 36439501 Free PMC article.
References
LinkOut - more resources
Full Text Sources

