Physiology-inspired bifocal fronto-parietal tACS for working memory enhancement
- PMID: 39315230
- PMCID: PMC11417162
- DOI: 10.1016/j.heliyon.2024.e37427
Physiology-inspired bifocal fronto-parietal tACS for working memory enhancement
Abstract
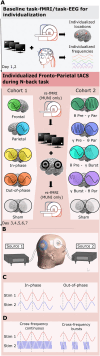
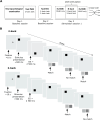
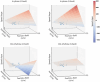
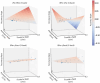
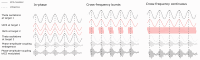
Aging populations face significant cognitive challenges, particularly in working memory (WM). Transcranial alternating current stimulation (tACS) offer promising avenues for cognitive enhancement, especially when inspired by brain physiology. This study (NCT04986787) explores the effect of multifocal tACS on WM performance in healthy older adults, focusing on fronto-parietal network modulation. Individualized physiology-inspired tACS applied to the fronto-parietal network was investigated in two blinded cross-over experiments. The first experiment involved monofocal/bifocal theta-tACS to the fronto-parietal network, while in the second experiment cross-frequency theta-gamma interactions between these regions were explored. Participants have done online WM tasks under the stimulation conditions. Network connectivity was assessed via rs-fMRI and multichannel electroencephalography. Prefrontal monofocal theta tACS modestly improved WM accuracy over sham (d = 0.30). Fronto-parietal stimulation enhanced WM task processing speed, with the strongest effects for bifocal in-phase theta tACS (d = 0.41). Cross-frequency stimulations modestly boosted processing speed with or without impairing task accuracy depending on the stimulation protocol. This research adds to the understanding of physiology-inspired brain stimulation for cognitive enhancement in older subjects.
Keywords: Cognition; Electric field modelling; Healthy aging; Multifocal; Neuroimaging; Orchestrated brain stimulation; Systems neuroscience; Working memory; tACS.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- GBD 2019 Demographics Collaborators Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160–1203. https://doi:10.1016/S0140-6736(20)30977-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

