Head-to-head comparison of two human papillomavirus vaccines for efficacy against cervical intraepithelial neoplasia grade 3 and adenocarcinoma in situ-population-based follow-up of two cluster-randomized trials
- PMID: 39315334
- PMCID: PMC11417099
- DOI: 10.3389/fcimb.2024.1437704
Head-to-head comparison of two human papillomavirus vaccines for efficacy against cervical intraepithelial neoplasia grade 3 and adenocarcinoma in situ-population-based follow-up of two cluster-randomized trials
Abstract
Introduction: We report head-to-head comparison of the bivalent and quadrivalent HPV vaccine efficacies against immediate precursors of cervical cancer from 15 years' country-wide cancer registry follow-up of phase III trial cohorts and an age-aligned cohort of unvaccinated women.
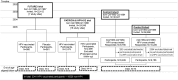
Methods: These individually and/or clusterrandomized cohorts of HPV6/11/16/18- and HPV16/18-vaccinated and unvaccinated women were enrolled, respectively, in 2002, 2004, and 2003/2005. The trial cohorts comprised initially 16- to 17-year-old HPV6/11/16/18-vaccinated FUTURE II (NCT00092534) participants (866) and HPV16/18-vaccinated PATRICIA (NCT00122681) and 012 trial (NCT00169494) participants (2,465), and 16,526 initially 16- to 19-year-old unvaccinated controls. After active 4-year clinical follow-up, passive, country-wide Finnish Cancer Registry (FCR) follow-up for cervical intraepithelial neoplasia grade 3 (CIN3) and adenocarcinoma in situ (AIS) was based on consented use of unique personal identifiers and started 6 months after the end of the FUTURE II and PATRICIA trials in 2007 and 2009, and ended at the end of 2019. The follow-up with altogether 229,020 follow-up years was age-aligned to ensure that similarly aged cohorts were passively followed up for 15 years post=vaccination for the intention-to-treat analyses of vaccine efficacy.
Results: Overall, we identified 5 and 16 CIN3 (no AIS) cases in the HPV6/11/16/18 and HPV16/18 cohorts, respectively, during the FCR-based follow-up. In the unvaccinated cohort, we identified 281 CIN3 cases, 20 AIS cases, and 13 cases with invasive cervical cancer. Vaccine efficacies against CIN3+ were 68.4% and 64.5% for the quadrivalent and the bivalent vaccines, respectively, with overlapping confidence intervals.
Discussion: Long-term follow-up of randomized, initially adolescent HPV-vaccinated and unvaccinated cohorts shows, in this head-to-head setting, that the bivalent and quadrivalent HPV vaccines are equally effective against immediate precursors of cervical cancer.
Keywords: cervical neoplasia; follow-up; human papillomavirus; randomized trial; vaccine efficacy.
Copyright © 2024 Lehtinen, Gray, Luostarinen, Eriksson, Apter, Bly, Harjula, Heikkilä, Hokkanen, Kuortti, Nieminen, Nummela, Paavonen, Palmroth, Petäjä, Pimenoff, Pukkala and Dillner.
Conflict of interest statement
DA, JD, ML, and JPaa have received grants for their HPV vaccination studies from Merck&Co. Inc. DA, JD, ML, and JPaa and GSK Biologicals DA, ML, JPaa. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

